Writing Binary Ionic Compounds Worksheet
Are you searching for a practical and interactive way to reinforce your knowledge of binary ionic compounds? Look no further! In this blog post, we will explore the benefits of using worksheets as an effective learning tool for mastering the concepts of entity and subject in binary ionic compounds. Whether you are a student aiming to improve your understanding or an educator in need of engaging resources for your classroom, this worksheet is designed to enhance your learning experience.
Table of Images 👆
- Writing Ionic Compound Formula Worksheet Answers
- Writing Ionic Compound Formula Worksheet Answers
- Naming Ionic Compounds Worksheet Answers
- Writing Formulas Criss Cross Method Worksheet
- Naming Ionic and Covalent Compounds Worksheet
- Writing Ionic Compound Formula Worksheet Answers
- Naming Ionic Compounds Worksheet Answer Key
- Writing Formulas Criss Cross Method Worksheet Answers
- Sodium and Sulfur Ionic Compound Formula
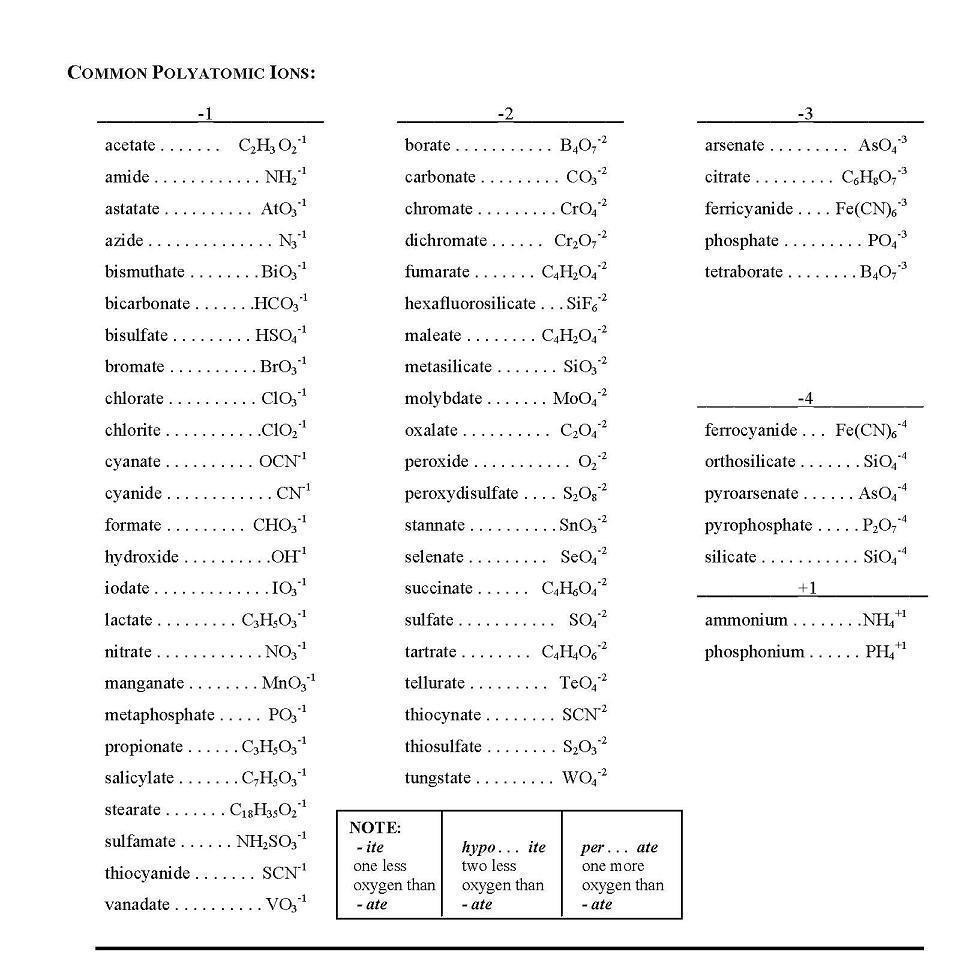
- Common Polyatomic Ion Table
- Protons Neutrons and Electrons Practice Worksheet Answers
- Protons Neutrons and Electrons Practice Worksheet Answers
- Protons Neutrons and Electrons Practice Worksheet Answers
- Protons Neutrons and Electrons Practice Worksheet Answers
- Protons Neutrons and Electrons Practice Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the purpose of this worksheet?
The purpose of this worksheet is to provide practice and reinforcement of specific skills or concepts, allowing the individual to develop a deeper understanding and proficiency in the subject matter.
What are binary ionic compounds?
Binary ionic compounds are compounds composed of positively charged metal cations and negatively charged nonmetal anions. These compounds are held together by ionic bonds, where the metal atom transfers one or more electrons to the nonmetal atom to achieve a stable electronic configuration. The resulting compound is electrically neutral, with the positive and negative charges balancing each other out.
How do you determine the formula of a binary ionic compound?
To determine the formula of a binary ionic compound, you need to identify the charges of the ions involved. The charges on the cation (positively charged ion) and anion (negatively charged ion) must balance out to achieve a neutral compound. The formula is then determined by cross-multiplying the charges and using them as subscripts for the ions. For example, in sodium chloride (NaCl), sodium (Na) has a +1 charge and chloride (Cl) has a -1 charge, resulting in a formula of NaCl.
What are cations and anions in binary ionic compounds?
In binary ionic compounds, cations are positively charged ions formed by atoms that have lost electrons, typically metals. Anions are negatively charged ions formed by atoms that have gained electrons, usually nonmetals. Cations and anions combine through ionic bonds to create compounds, with the cation attracting the anion due to their opposite charges.
What is the role of the cation in the formula of a binary ionic compound?
The cation in the formula of a binary ionic compound provides the positive charge necessary to balance the negative charge of the anion. This allows for the compound to be electrically neutral overall. The cation is typically a metal ion that has lost one or more electrons, resulting in a positive charge.
How do you determine the charge of a cation or anion?
The charge of a cation or anion can be determined by looking at the number of electrons it has gained or lost. Cations are formed when an atom loses electrons, resulting in a positive charge, while anions are formed when an atom gains electrons, resulting in a negative charge. The charge of a cation or anion is also typically indicated by a Roman numeral in parentheses following the name of the element to denote the charge.
What are some examples of binary ionic compounds?
Some examples of binary ionic compounds include sodium chloride (NaCl), magnesium oxide (MgO), potassium iodide (KI), and calcium fluoride (CaF2).
How do you write the chemical formulas for binary ionic compounds?
To write the chemical formulas for binary ionic compounds, you first need to determine the charges of the ions involved. The cation (positively charged ion) typically comes from a metal, while the anion (negatively charged ion) comes from a nonmetal. Use the crisscross method, where you cross the charges of the ions to determine the subscripts in the formula. Simplify the subscripts if necessary to get the simplest ratio of ions. Finally, write the chemical formula with the cation first followed by the anion.
What are the rules for naming binary ionic compounds?
Binary ionic compounds are named by combining the names of the cation and anion. The cation (metal) keeps its elemental name, while the anion (non-metal) ending is typically changed to "-ide." For example, in NaCl, sodium chloride, the sodium cation combines with the chlorine anion. When the cation has more than one possible oxidation state, Roman numerals are used in parenthesis to indicate the charge of the cation.
What are some common mistakes to avoid when writing and naming binary ionic compounds?
Some common mistakes to avoid when writing and naming binary ionic compounds include not correctly identifying the ions involved, not balancing the charges of the ions to achieve a neutral compound, and not using the appropriate prefixes for the elements involved. It is crucial to remember that the cation (positive ion) comes first and the anion (negative ion) comes second in naming binary ionic compounds. Additionally, make sure to refer to the periodic table to determine the charges of the ions and use the correct suffixes (-ide) for the anions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.































Comments