Worksheets States of Matter Sorting
If you are teaching elementary school students about the different states of matter, you may find it useful to use worksheets to reinforce their understanding. Worksheets can help students practice sorting different objects into their respective states of matter, allowing them to exercise their understanding of this scientific concept.
Table of Images 👆
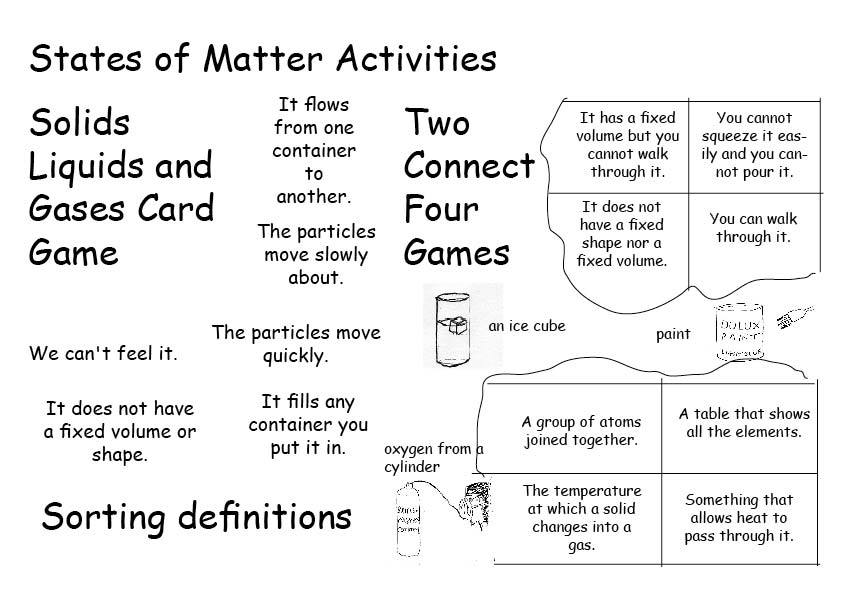
- States of Matter Activities
- States of Matter Worksheets
- States of Matter Worksheets Middle School
- States of Matter Worksheets Kindergarten
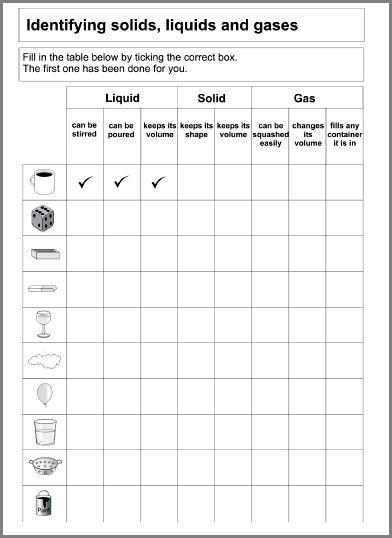
- States of Matter Matching Worksheet
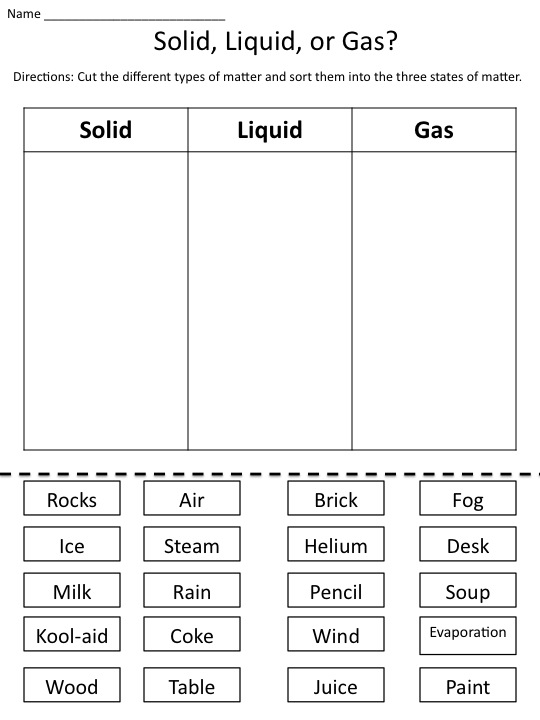
- States of Matter Sorting Activity
- States of Matter Worksheets 5th Grade
- States of Matter Worksheets 2nd Grade
- States of Matter Printables
- States of Matter Cut and Paste Worksheet
- States of Matter Activities
- States of Matter Worksheet Answers
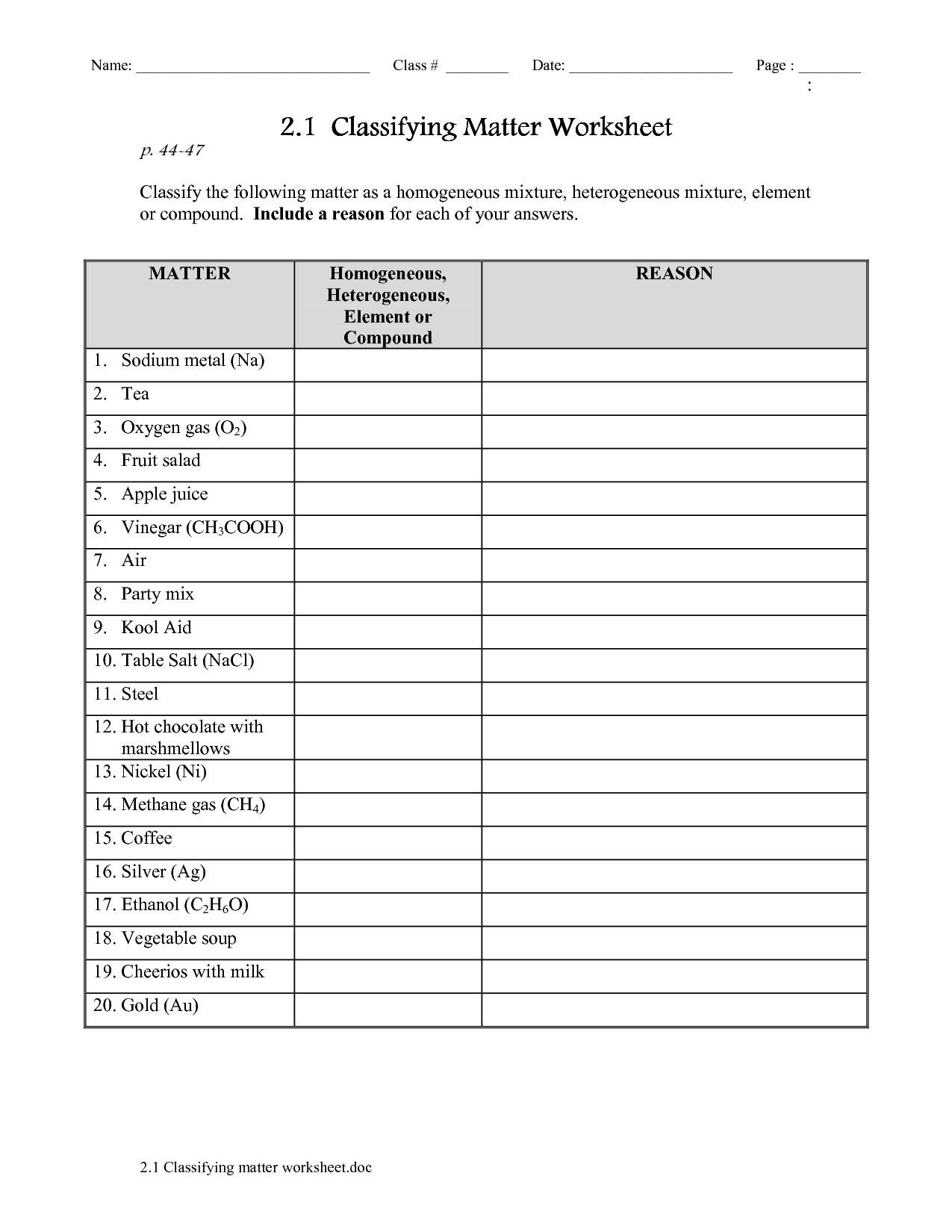
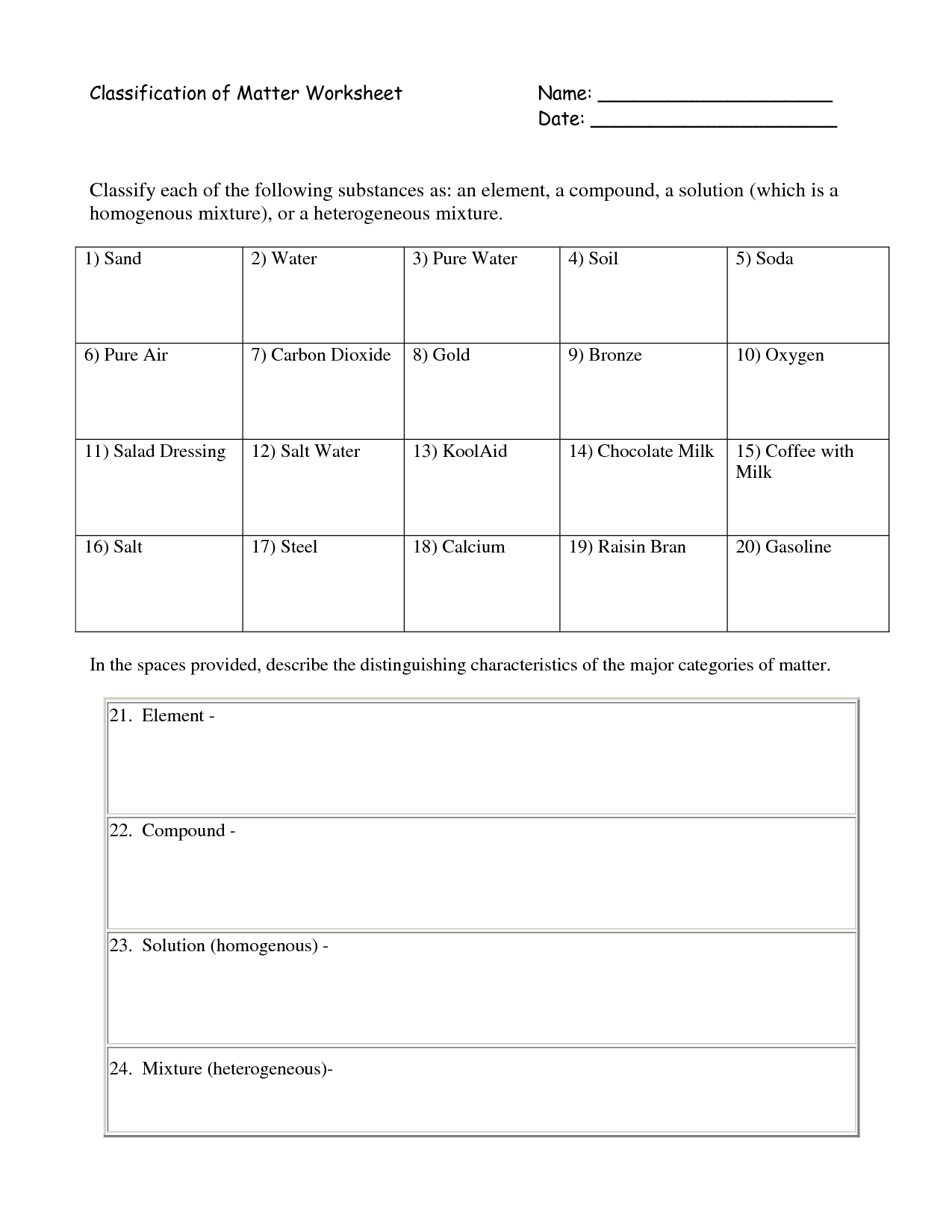
- Classifying Matter Worksheet Answers
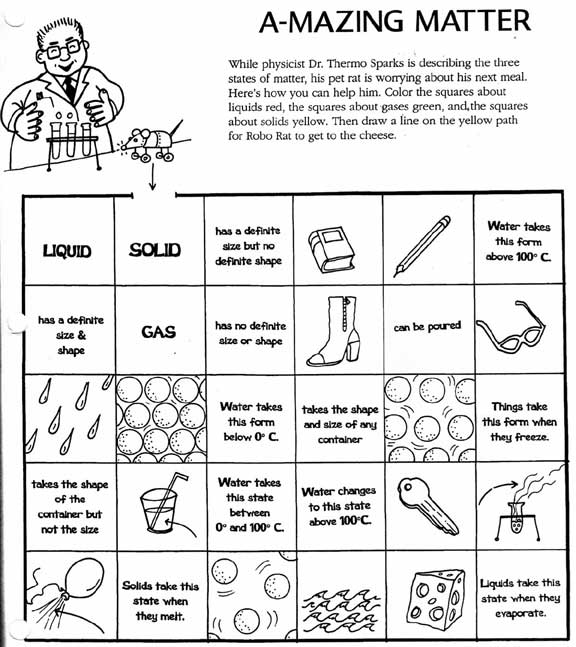
- State of Matter Gas Liquid and Solids Worksheets
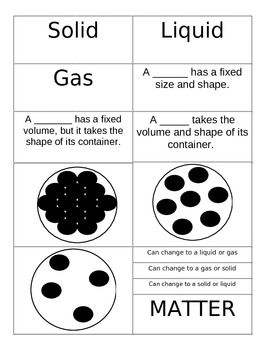
- Properties of Matter Sort
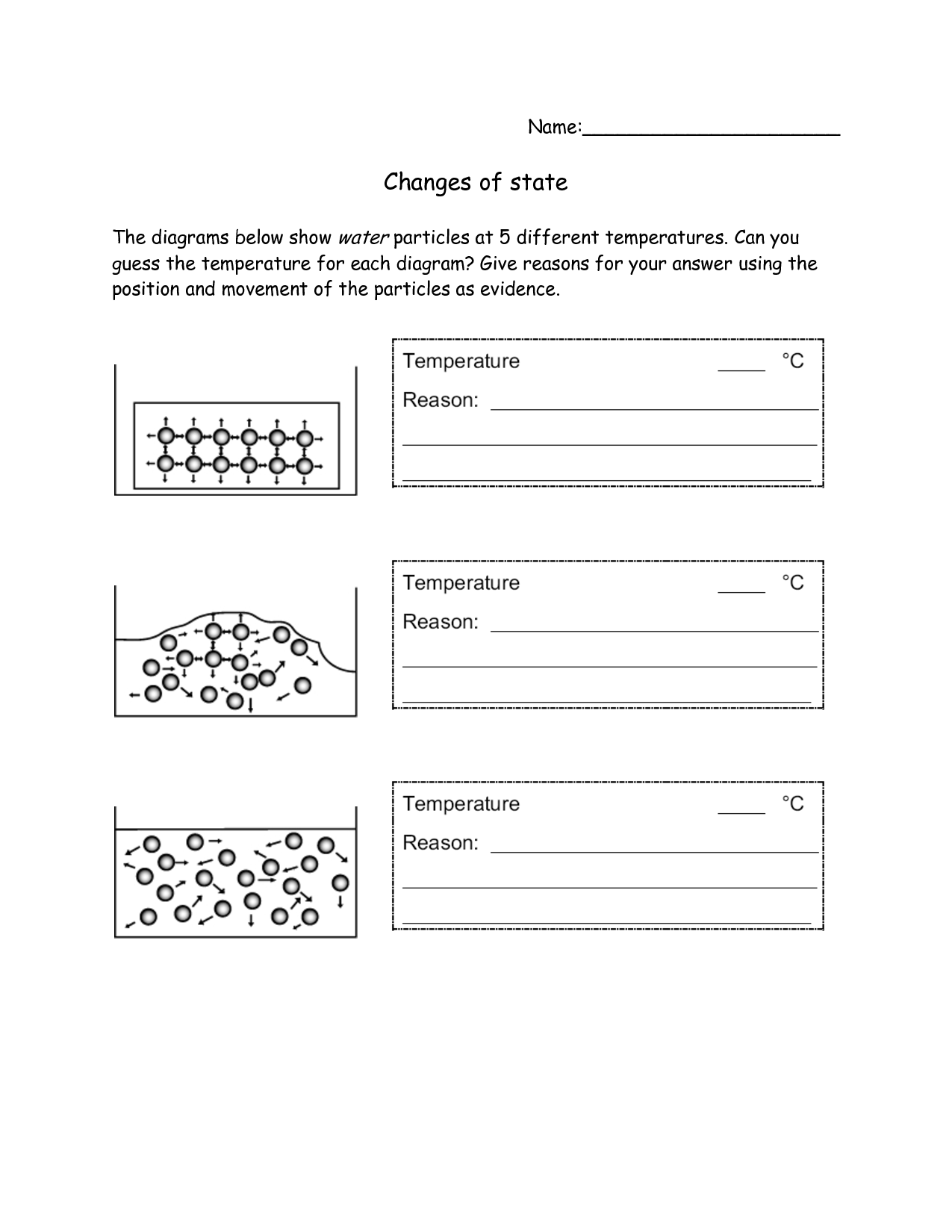
- States of Matter Changes Worksheet
- Matter Solid-Liquid Gas Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
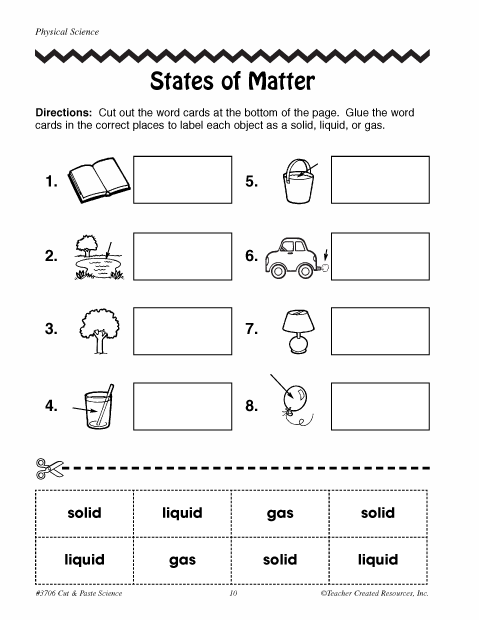
What are the three main states of matter?
The three main states of matter are solid, liquid, and gas. Solid has a definite shape and volume, liquid has a definite volume but takes the shape of its container, and gas has neither a definite shape nor volume, filling the entire space available to it.
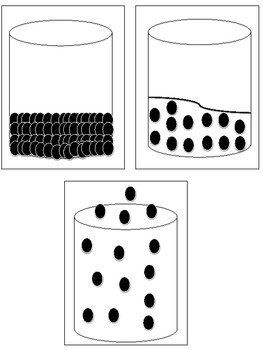
How does the arrangement of particles differ in solids, liquids, and gases?
In solids, particles are tightly packed together in a regular and fixed arrangement, with little movement. In liquids, particles are still close together but can move past each other, allowing the liquid to flow. In gases, particles are far apart and move freely and rapidly, filling the space of their container.
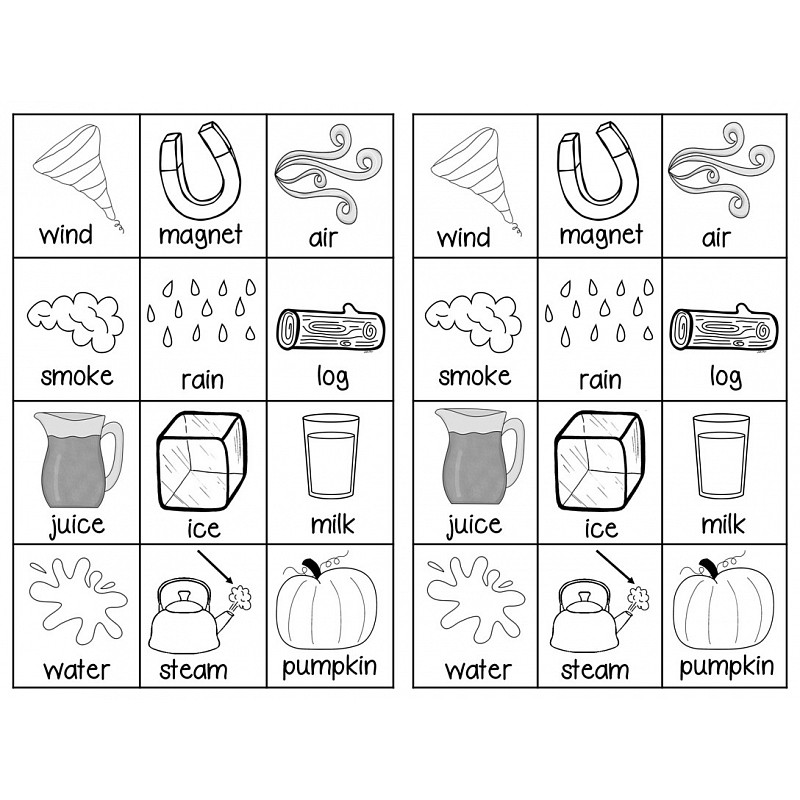
What are some examples of solids?
Some examples of solids include wood, metal, plastic, stone, glass, ice, and ceramic.
Describe the shape and volume of a liquid.
A liquid takes the shape of its container, meaning it has no fixed shape of its own. It assumes the shape of the bottom of the container it is poured into. Additionally, a liquid has a fixed volume, meaning it does not compress easily and maintains a constant volume regardless of the shape of its container.
How do particles in a gas behave?
Particles in a gas move randomly and freely in all directions, colliding with each other and with the walls of their container. They have high kinetic energy and are widely spaced apart, leading to a lack of strong attractive forces between them. This behavior allows gas particles to fill the entire volume of their container and provides them with the ability to expand and contract to fit the available space.
Give an example of a gas.
Oxygen is an example of a gas.
Can matter change from one state to another?
Yes, matter can change from one state to another through processes like melting, freezing, evaporation, and condensation. These changes happen when the temperature or pressure of the matter is altered, causing its particles to move differently and transition from one state (solid, liquid, gas) to another.
What is the process called when a solid changes directly into a gas?
The process is called sublimation, where a solid substance transforms directly into a gas without passing through the liquid phase.
How does energy affect the state of matter?
Energy can affect the state of matter by changing its temperature. When energy is added to a substance, the particles within the substance gain kinetic energy and move faster, causing the substance to heat up and potentially change state. For example, adding energy to a solid can melt it into a liquid, and adding more energy can turn the liquid into a gas. Conversely, removing energy from a substance can cause it to lose heat and transition to a lower energy state, such as a liquid cooling into a solid.
What is the role of temperature in changing the state of matter?
Temperature plays a crucial role in changing the state of matter by providing the energy required for particles to overcome intermolecular forces and transition between solid, liquid, and gas states. Increasing temperature typically causes particles to move more energetically, leading to melting (solid to liquid), vaporization (liquid to gas), or sublimation (solid to gas). Conversely, decreasing temperature causes particles to slow down, resulting in freezing (liquid to solid) or condensation (gas to liquid). This relationship between temperature and the state of matter is key to understanding phase transitions in physical systems.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments