Worksheet Elements and Bonding
Worksheets are an essential tool for students who are studying the concepts of elements and bonding. These valuable resources provide a structured way to practice and reinforce understanding of this fundamental topic in chemistry. By engaging with worksheets, students can deepen their knowledge of elemental properties, types of bonding, and the factors that influence chemical interactions. Whether you're a high school student preparing for exams or an undergraduate pursuing a degree in science, worksheets can be an effective means to grasp these core concepts.
Table of Images 👆
- Ionic and Covalent Bonding Worksheet
- Chemical Elements Worksheets
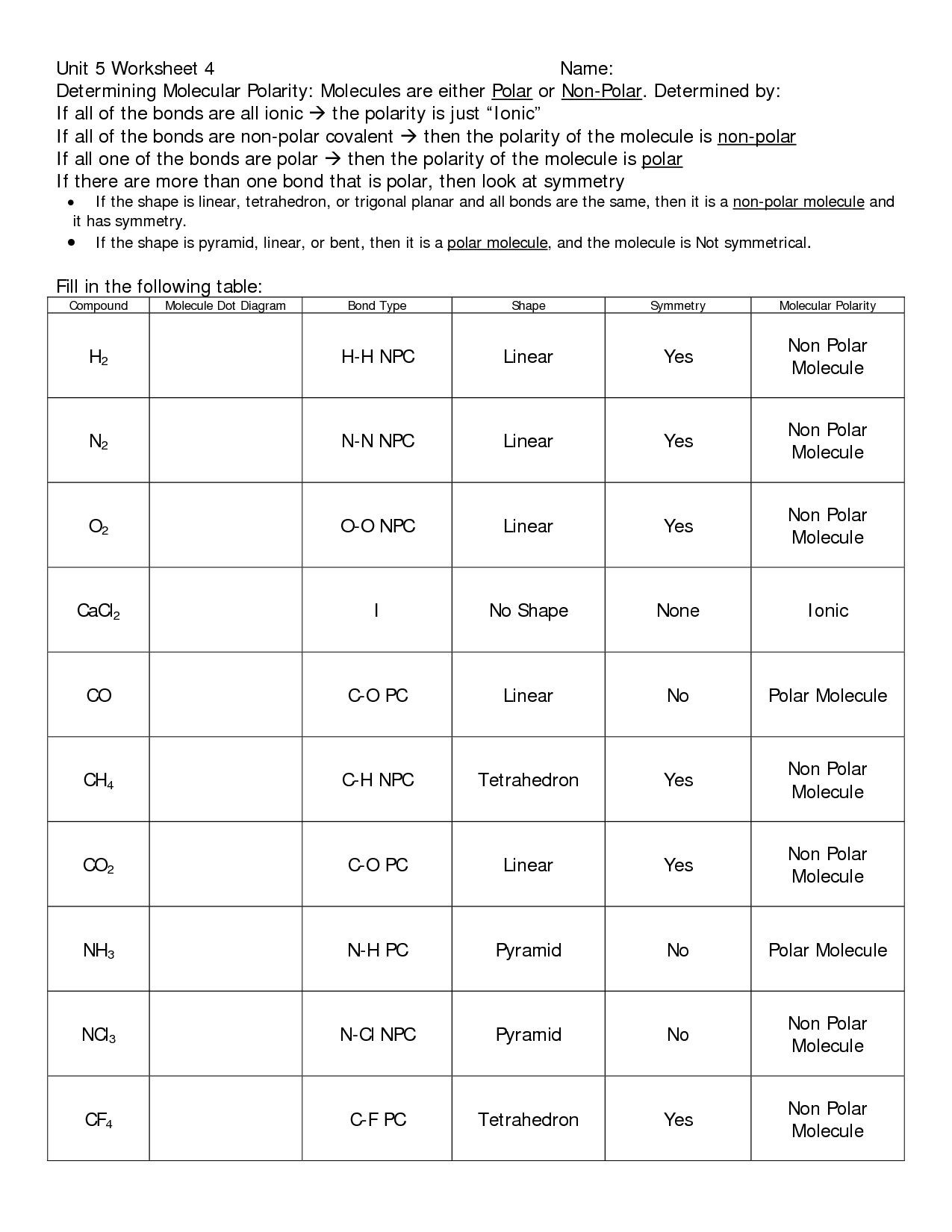
- Elements and Bonding Worksheet Answers
- Lewis Dot Covalent Bond Worksheet
- Naming Covalent Compounds Worksheet
- Atomic Structure Worksheet Answers
- Atoms Bonding and Covalent Bond Worksheet
- Ionic and Covalent Bonding Worksheet
- Chapter 6 Chemical Bonding Worksheet Answers
- Chemical Bonding Worksheet Answer Key
- Atoms and Molecules in Chemical Formulas Worksheet
- Chemical Bonds Worksheet Answers
- Atoms and Ions Worksheet Answer Key
- Naming Molecular Compounds Worksheet
- Ionic Bonding Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
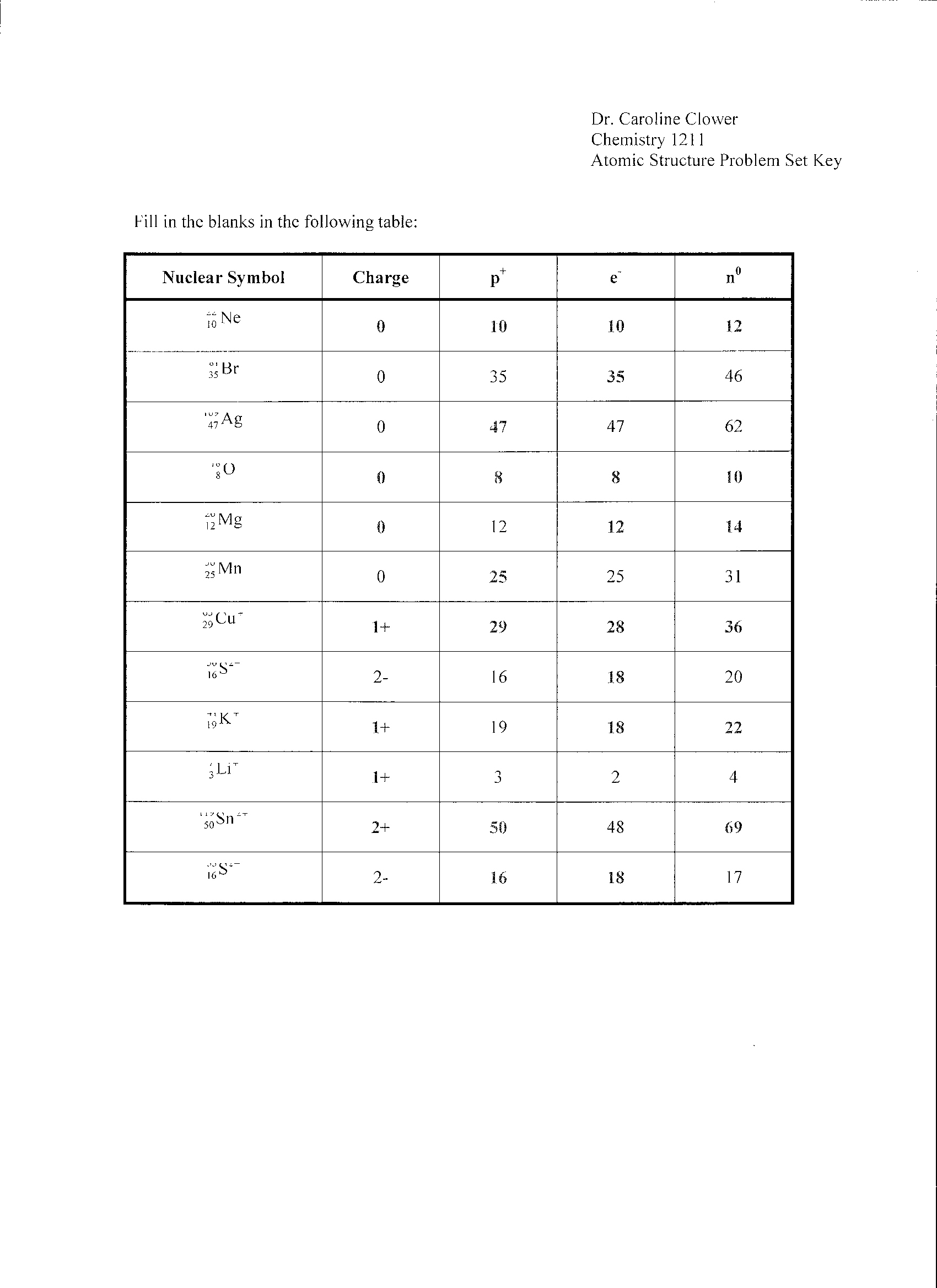
What is an atom?
An atom is the smallest unit of matter that retains the properties of an element, consisting of a nucleus containing protons and neutrons, surrounded by electrons in orbitals.
What are valence electrons?
Valence electrons are the electrons located in the outermost energy level of an atom. These electrons are involved in the formation of chemical bonds and determine the reactivity and bonding behavior of an atom. The number of valence electrons an atom has can help predict its reactivity and the types of chemical bonds it can form with other atoms.
What is an ion?
An ion is an atom or molecule that has an unequal number of electrons compared to protons, leading to a net positive or negative electrical charge. Positively charged ions are called cations, while negatively charged ions are called anions. Ions play a crucial role in various chemical reactions and physiological processes in living organisms.
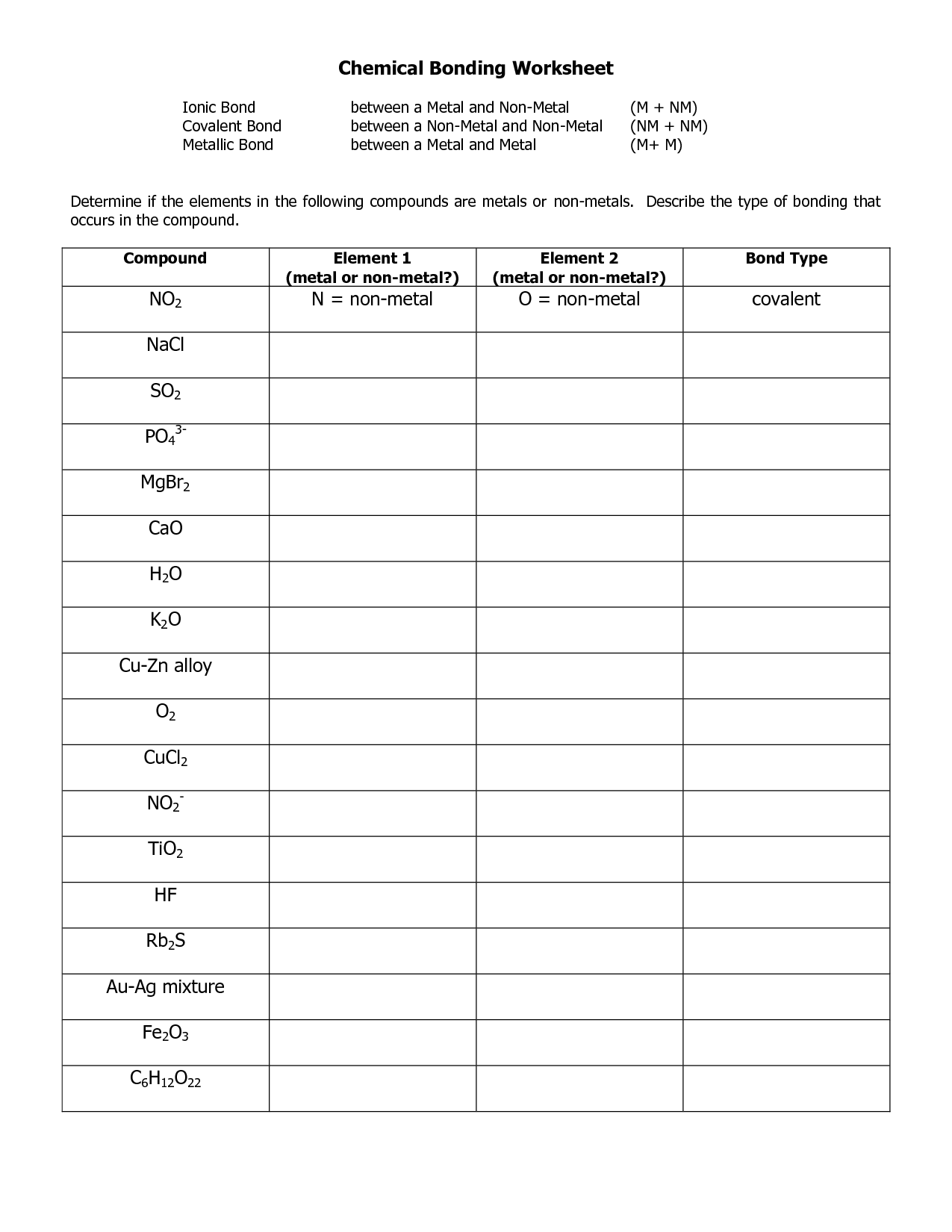
What is an ionic bond?
An ionic bond is a type of chemical bond that forms between two atoms when one atom transfers electrons to another atom. This transfer of electrons results in the formation of positively charged ions and negatively charged ions, which are then attracted to each other due to their opposite charges, creating a strong bond between the atoms.
What is a covalent bond?
A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. It is characterized by the atoms mutually sharing electrons to achieve a more stable electron configuration. This type of bond is commonly found in molecules and compounds composed of nonmetal elements.
What is a polar covalent bond?
A polar covalent bond is a type of chemical bond where electrons are unequally shared between two atoms, resulting in a slight positive and negative charge on each atom. This unequal sharing occurs because one atom has a higher electronegativity than the other, causing it to attract electrons more strongly. This results in a partial separation of charges, with one end of the bond being slightly positive and the other slightly negative.
What is a metallic bond?
A metallic bond is a type of chemical bond that occurs between atoms in a metallic element. In a metallic bond, the atoms within a metal lattice share a collective pool of electrons that are free to move throughout the entire structure, giving metals their characteristic properties such as high electrical and thermal conductivity, ductility, and malleability.
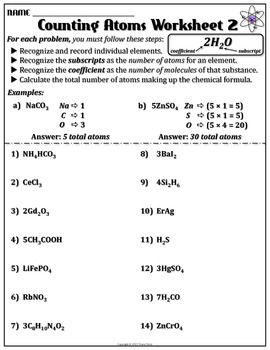
What is a chemical formula?
A chemical formula is a concise way to represent the types and numbers of atoms present in a compound. It uses symbols for elements and subscripts to indicate the number of each element in one molecule of the compound..forChildioxide, for example, the chemical formula is CO2, which means one carbon atom and two oxygen atoms are present in each molecule of the compound.
What is an empirical formula?
An empirical formula is the simplest whole-number ratio of atoms in a compound. It shows the relative number of atoms of each element in a compound, without specifying the actual number of atoms present. Empirical formulas are determined based on experimental data, like the mass or percentage composition of the elements in a sample.
What is a Lewis structure?
A Lewis structure is a diagram that represents the arrangement of valence electrons in a molecule. It consists of symbols for the atoms and lines or dots to show the sharing or transfer of electrons between atoms, helping to illustrate the connectivity and bonding in a compound. Lewis structures are used in chemistry to understand the chemical properties and behavior of molecules.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments