Worksheet 3.4 Answers for Chemistry
Chemistry students who are seeking answers to Worksheet 3.4 can find the solution they need right here. This blog post provides an easy-to-follow guide that covers the entity and subject matter of Worksheet 3.4 in Chemistry.
Table of Images 👆
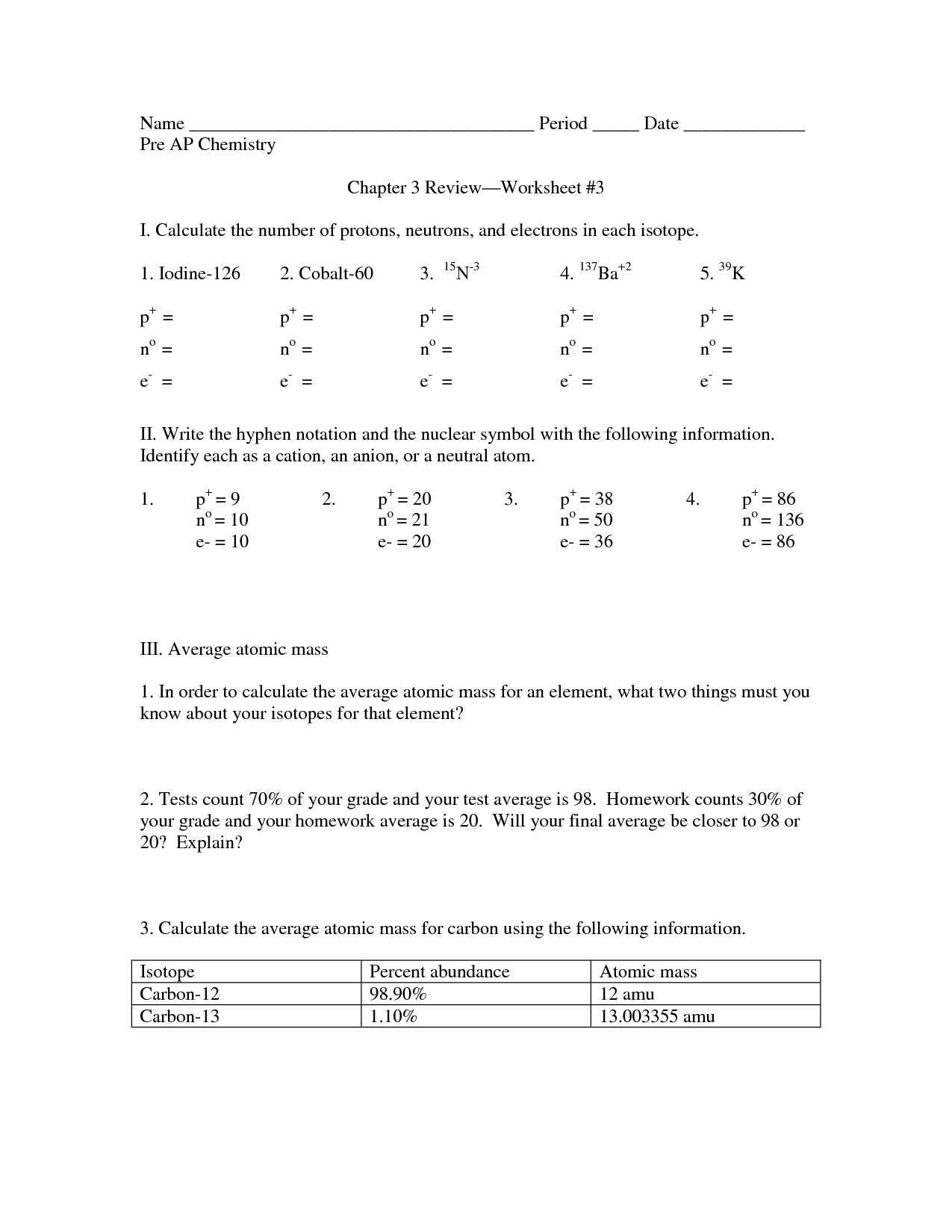
- Chemistry Worksheet Isotope Notation Answers
- Chemistry Unit 5 Worksheet 2 Answer Key
- Solubility Curves Worksheet Answers
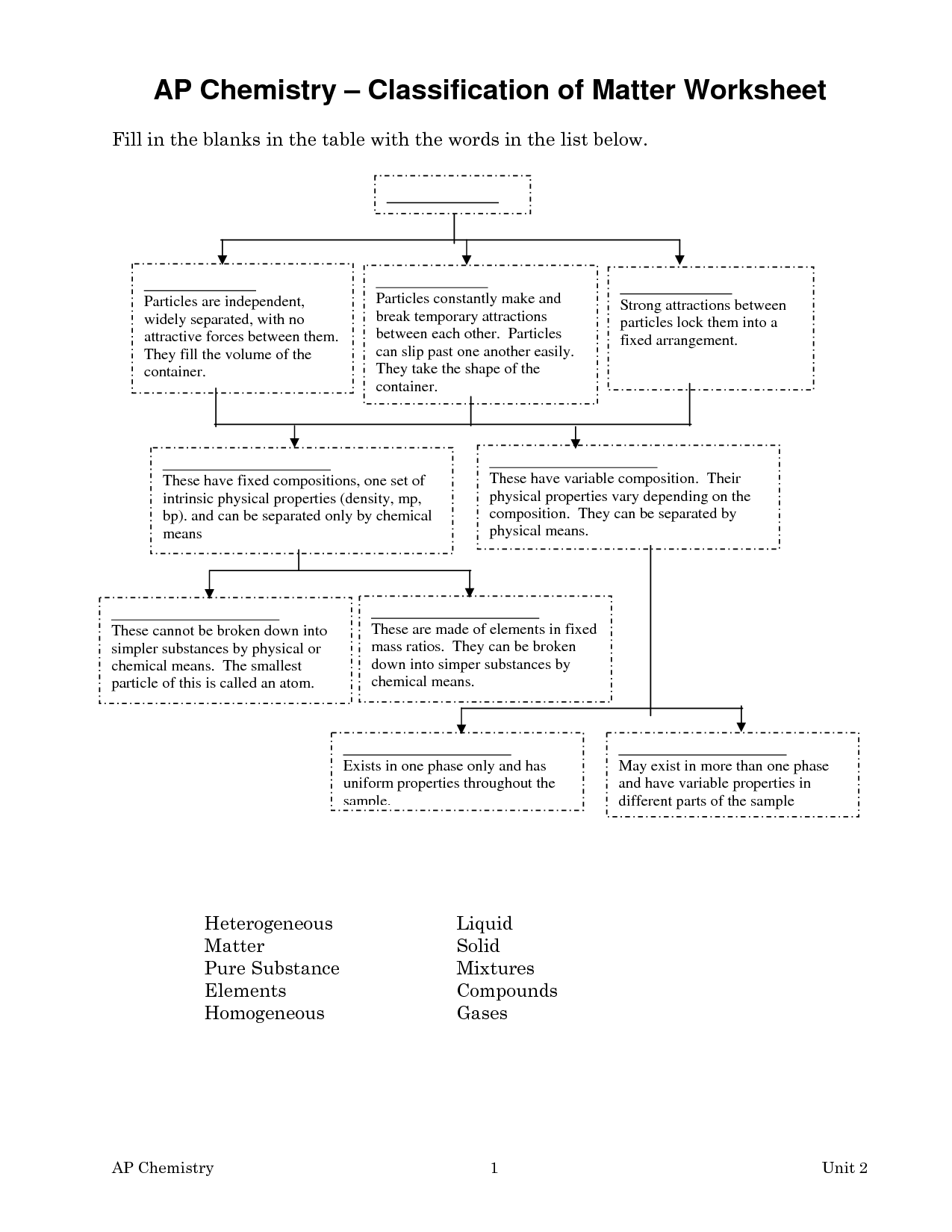
- Chemistry Worksheet Matter 1 Answer Key
- Solubility Curve Worksheet Answer Key
- Naming Organic Compounds Worksheet Answer
- Balancing Chemical Equations Worksheet 1
- Dimensional Analysis Worksheet Answers
- Chemistry Stoichiometry Worksheet Answer Key
- Chemistry Worksheet Answer Keys
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the chemical formula for water? H2O.
H2O.
What is the atomic number of carbon? 6.
Correct, the atomic number of carbon is 6.
What is the difference between an element and a compound? An element is a substance made up of only one type of atom, while a compound is a substance made up of two or more different types of atoms chemically bonded together.
An element is a substance made up of only one type of atom, while a compound is a substance made up of two or more different types of atoms chemically bonded together.
What is the pH scale used to measure? The pH scale measures the acidity or alkalinity of a solution.
The pH scale measures the acidity or alkalinity of a solution.
What is Avogadro's number? Avogadro's number is 6.022 × 10^23 and represents the number of particles (atoms, molecules, or ions) in one mole of a substance.
Avogadro's number is 6.022 × 10^23 and represents the number of particles (atoms, molecules, or ions) in one mole of a substance.
What is the octet rule? The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with a full outer shell of eight electrons.
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with a full outer shell of eight electrons.
What is the definition of a mole in chemistry? A mole is a unit of measurement that represents the amount of a substance containing the same number of particles (atoms, molecules, or ions) as there are atoms in 12 grams of carbon-12.
A mole is a unit of measurement in chemistry that represents the amount of a substance containing the same number of particles (atoms, molecules, or ions) as there are atoms in 12 grams of carbon-12.
What is the difference between an exothermic and an endothermic reaction? An exothermic reaction releases energy in the form of heat, while an endothermic reaction absorbs energy from the surroundings.
Exothermic reactions release energy in the form of heat, while endothermic reactions absorb energy from the surroundings.
What is the ideal gas law equation? The ideal gas law equation is PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
The ideal gas law equation is PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
What is the difference between a physical change and a chemical change? A physical change is a change in the physical properties of a substance without a change in its chemical composition, while a chemical change involves the breaking and forming of chemical bonds to produce new substances with different chemical compositions.
A physical change is a change in the physical properties of a substance without a change in its chemical composition, while a chemical change involves the breaking and forming of chemical bonds to produce new substances with different chemical compositions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments