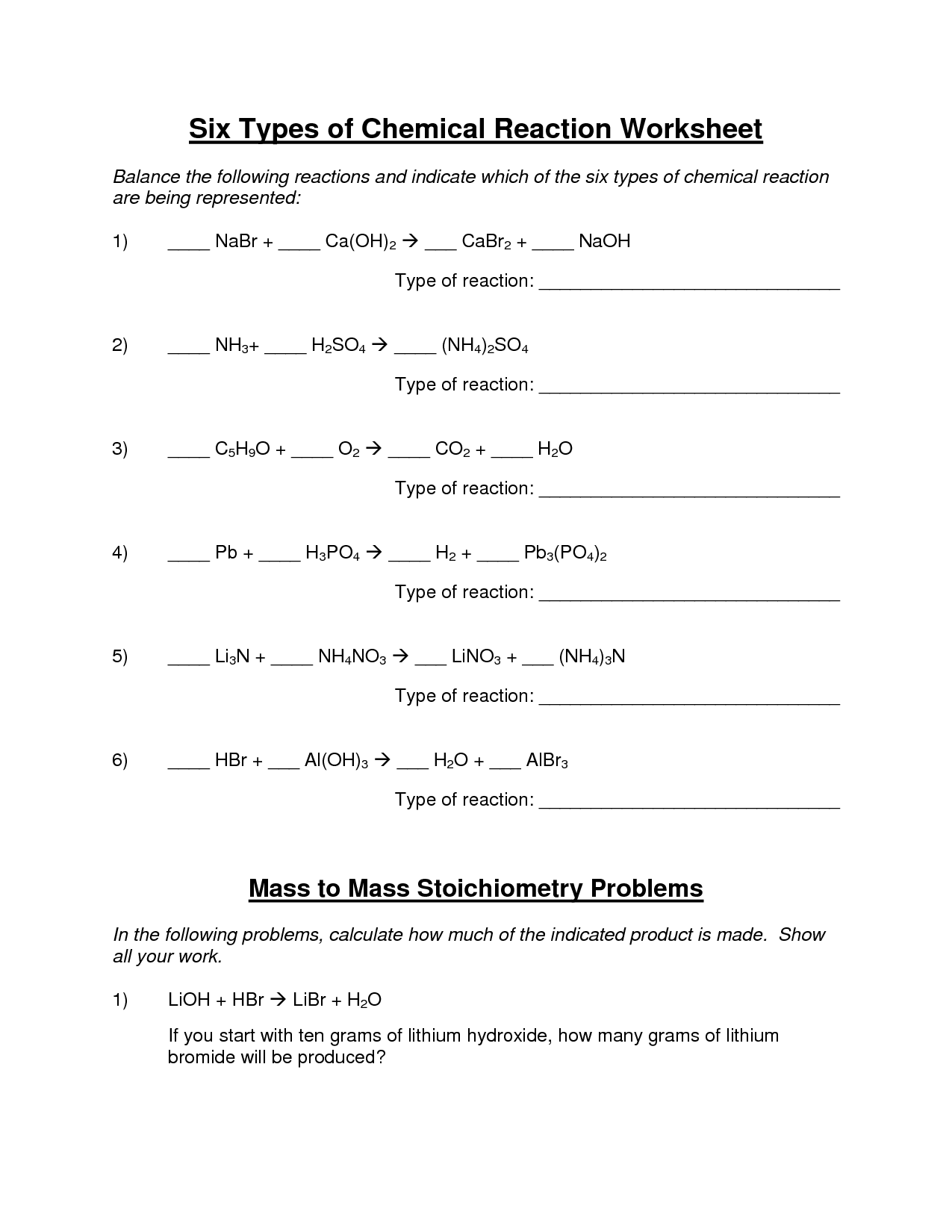
Types of Reactions Worksheet
In order to effectively understand and practice different types of reactions, a worksheet can be an invaluable tool. Worksheets provide an organized and structured way to learn and reinforce knowledge, making them ideal for students and learners who prefer a hands-on approach to studying chemistry. With clear instructions and examples, a reactions worksheet ensures that individuals can master the concepts and confidently apply them in various scenarios.
Table of Images 👆
- Types of Chemical Reactions Worksheet Answer Key
- Types of Chemical Reactions Worksheet Answers
- Types of Chemical Reactions Worksheet Answer Key
- Identifying Chemical Reaction Types Worksheet
- Classifying Chemical Reactions Worksheet
- Chemical Reaction Types Worksheet
- Classifying Chemical Reactions Worksheet Answer Key
- Types of Chemical Reactions Worksheet Answers
- Double Replacement Reaction Worksheet Answers
- Double Displacement Reaction Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
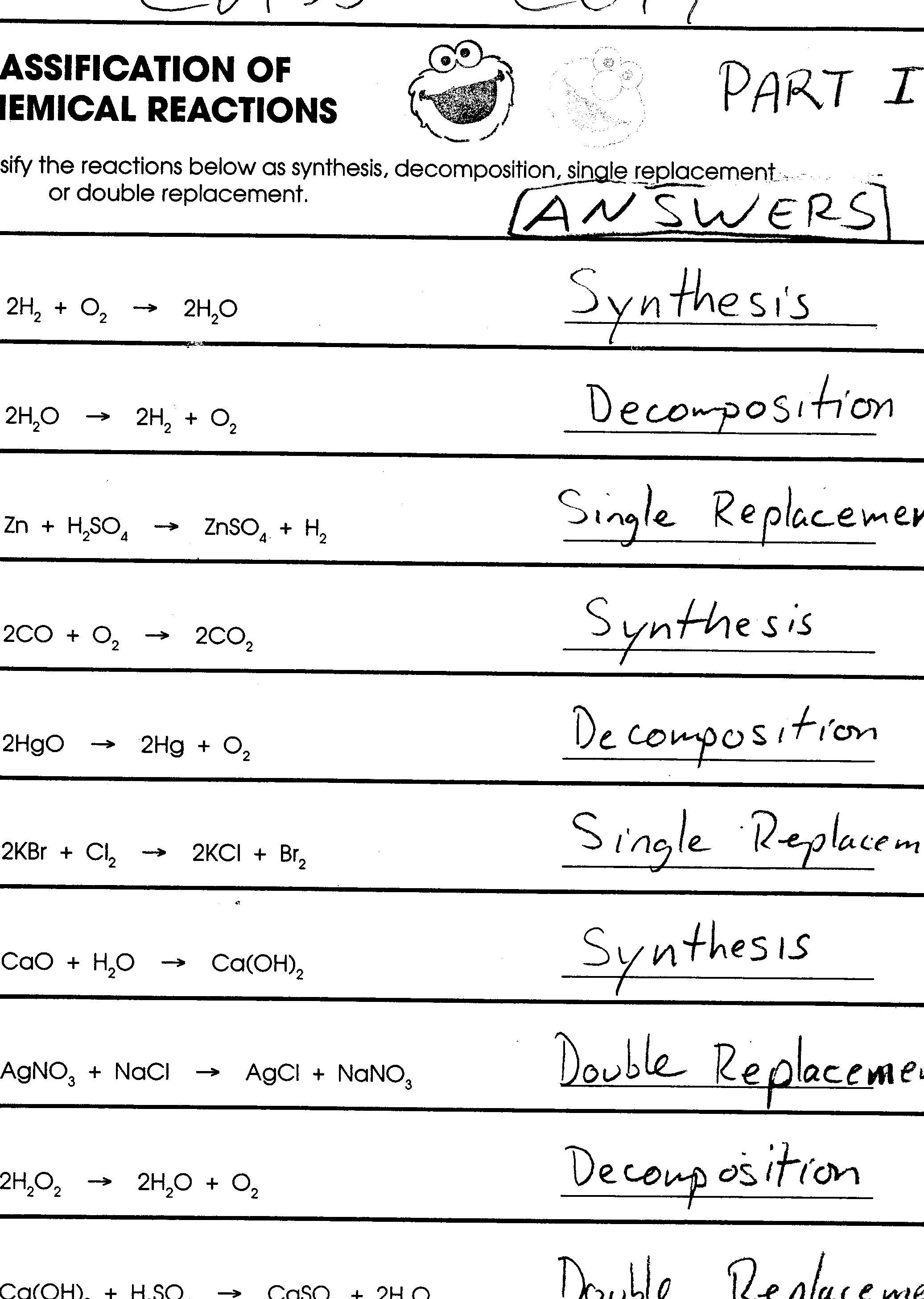
What is a synthesis reaction?
A synthesis reaction is a chemical reaction in which two or more simple substances combine to form a more complex product. This type of reaction typically involves the formation of a single product and is characterized by the general equation: A + B --> AB.
What is a decomposition reaction?
A decomposition reaction is a type of chemical reaction where a compound breaks down into simpler substances or elements. This can be triggered by heat, light, electricity, or certain chemicals, causing the compound to split apart into its constituent parts through various chemical processes.
What is a single replacement reaction?
A single replacement reaction, also known as a displacement reaction, is a type of chemical reaction where an element replaces another element in a compound. This occurs when a more reactive element displaces a less reactive element from a compound, resulting in the formation of a new compound and a different element.
What is a double replacement reaction?
A double replacement reaction is a chemical reaction in which two compounds exchange ions to form two new compounds. This type of reaction typically occurs when two ionic compounds are mixed together in an aqueous solution. The cations and anions switch partners to produce two new compounds, each with a combination of ions from the original compounds. Double replacement reactions are also known as double displacement reactions or metathesis reactions.
What is a combustion reaction?
A combustion reaction is a chemical reaction that involves the rapid oxidation of a substance, typically a hydrocarbon, in the presence of oxygen to produce heat, light, and usually carbon dioxide and water as byproducts. This process is exothermic, meaning it releases energy in the form of heat and light.
What is an oxidation-reduction reaction?
An oxidation-reduction reaction, also known as a redox reaction, is a type of chemical reaction where one substance loses electrons (oxidation) while another substance gains those electrons (reduction). In this process, the oxidized substance increases its oxidation state, while the reduced substance decreases its oxidation state. This transfer of electrons leads to the formation of new compounds and the release of energy.
What is a precipitation reaction?
A precipitation reaction is a chemical reaction in which two soluble ionic compounds react to form an insoluble solid compound, known as a precipitate. This usually occurs when two solutions are mixed together and a solid substance forms due to the limited solubility of the resulting compound, causing it to precipitate out of the solution.
What is a neutralization reaction?
A neutralization reaction is a chemical reaction between an acid and a base that results in the formation of water and a salt. The acid and base neutralize each other, leading to a decrease in the acidity or alkalinity of the solution as they combine to form water and a salt compound.
What is a redox reaction?
A redox reaction, or reduction-oxidation reaction, is a type of chemical reaction that involves the transfer of electrons between two chemical species. One species loses electrons (oxidation) while another gains electrons (reduction). This transfer of electrons results in a change in the oxidation states of the elements involved in the reaction.
What is a displacement reaction?
A displacement reaction is a chemical reaction in which one element displaces another element from a compound in solution, based on the reactivity of the elements involved. This occurs when a more reactive element replaces a less reactive element in a compound, resulting in the formation of a new compound and a different element being released.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.










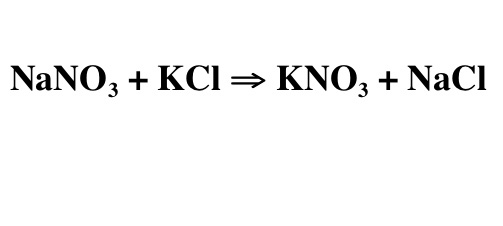















Comments