States of Matter Worksheet Answer Key
The States of Matter Worksheet Answer Key provides a comprehensive solution to help students better understand the different states of matter. Designed for learners in upper elementary grades or middle school, this answer key is a valuable resource to reinforce knowledge and comprehension about the properties and characteristics of solids, liquids, and gases. With clear and concise explanations, students can confidently grasp the concepts and apply them in various scientific scenarios.
Table of Images 👆
- Chapter 13 States of Matter Worksheet Answers
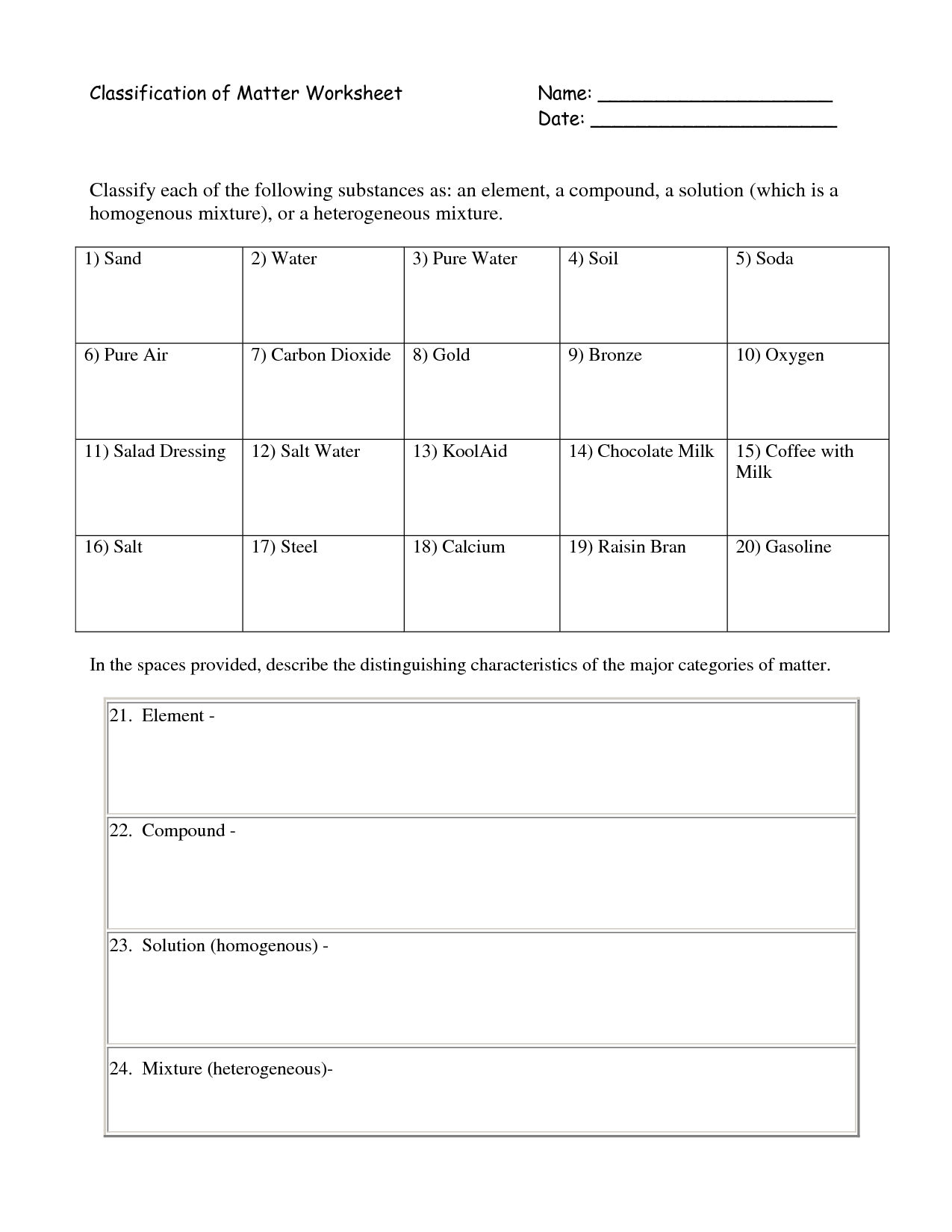
- Classification of Matter Worksheet Answers
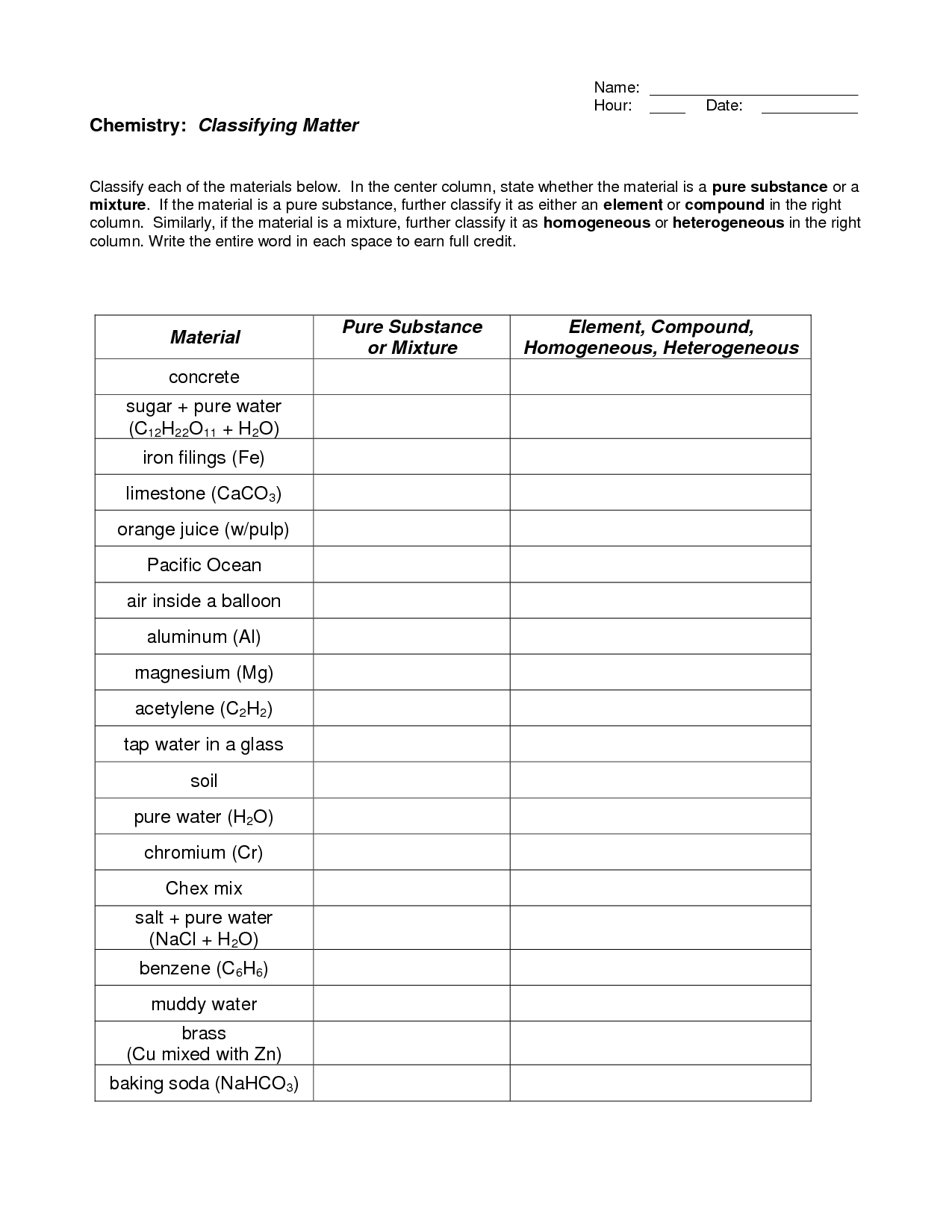
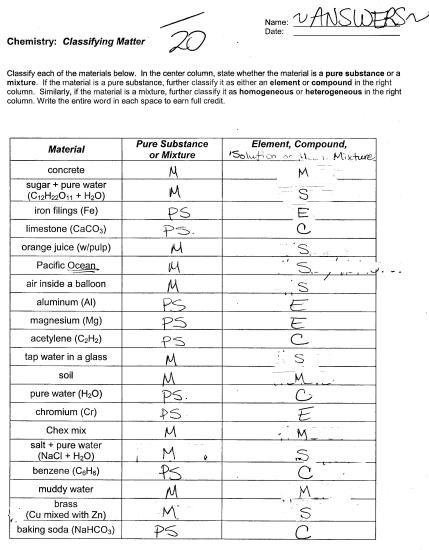
- Classifying Matter Worksheet Answers
- Chemistry Review Answers Chapter 10
- Classifying Matter Worksheet Answers
- Properties of Water Worksheet Answer Key
- Classification of Matter Worksheet Answers
- States of Matter Study Guide Answers
- Properties of Matter Worksheet Answers
- States of Matter Crossword Puzzle Answers
- Classifying Matter Worksheet Answers
- Matter Substances vs Mixtures Worksheet
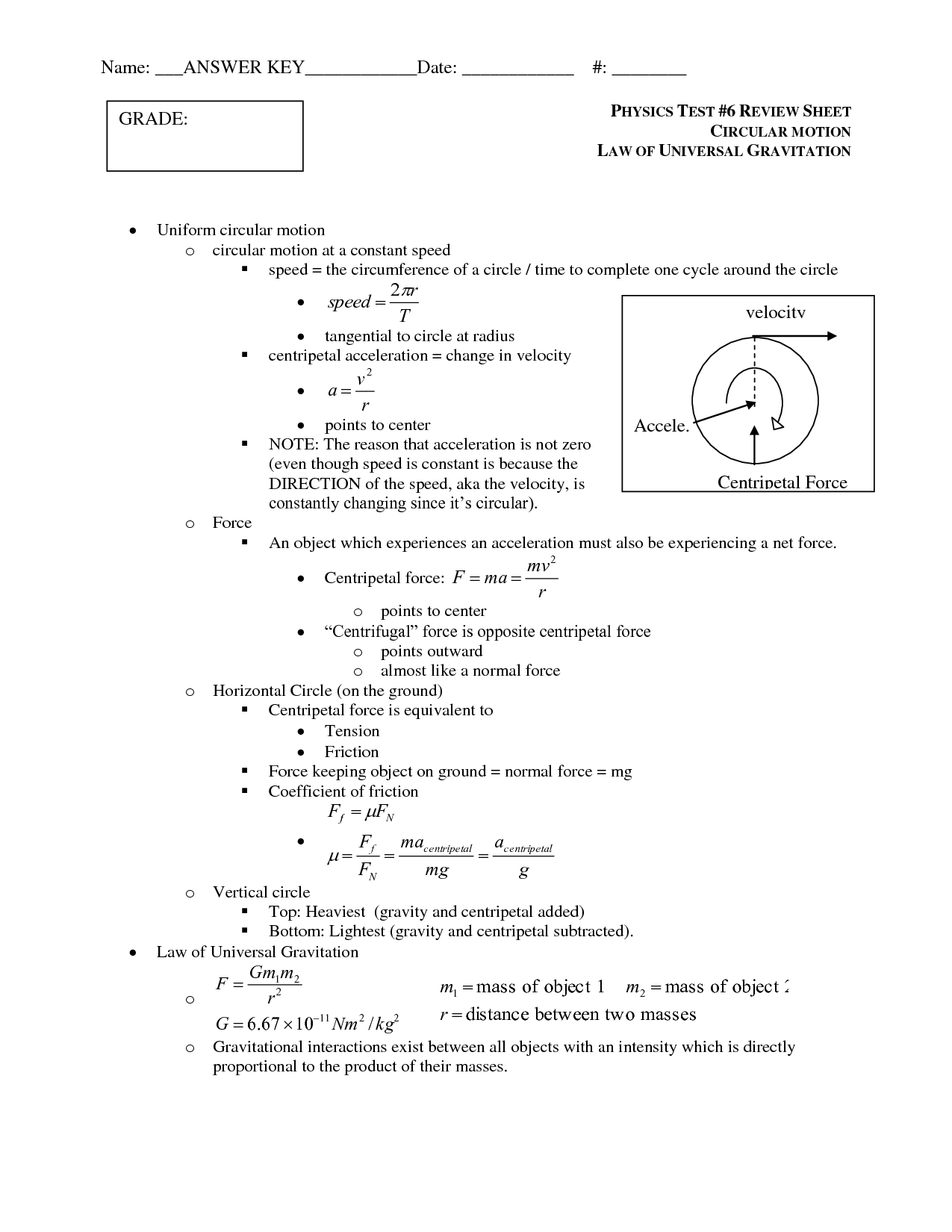
- Universal Gravitation PhET Lab Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What are the three states of matter? - Solid, liquid, and gas.
Yes, the three states of matter are solid, liquid, and gas.
In which state of matter do particles have the least amount of energy? - Solid.
Solid.
How are the particles arranged in a solid? - Closely packed together in a regular pattern.
In a solid, particles are arranged closely packed together in a regular pattern. This results in a fixed shape and volume for the solid.
What happens to the shape and volume of a liquid when it is poured into a different container? - The shape changes to fit the new container, but the volume remains the same.
When a liquid is poured into a different container, its shape changes to fit the new container's dimensions, but the volume of the liquid remains constant. The molecules of the liquid adjust their arrangement to conform to the shape of the new container, allowing the liquid to take on a different form while still occupying the same amount of space.
Which state of matter has the most energy? - Gas.
Gas has the most energy among the three states of matter - solid, liquid, and gas. Gas molecules have a higher amount of kinetic energy as they move freely and rapidly in all directions, compared to the more tightly packed molecules in liquids and solids.
Do gas particles have definite shape and volume? - No, they take the shape and volume of their container.
Yes
What is condensation? - The process by which a gas changes into a liquid.
Yes, condensation is the process by which a gas changes into a liquid as a result of cooling or compression, causing the gas molecules to lose kinetic energy and come together to form a liquid.
Define sublimation. - The process in which a solid changes directly into a gas without going through the liquid state.
Sublimation is the process in which a solid changes directly into a gas without transitioning through the liquid state. This means that when a substance undergoes sublimation, it goes from a solid to a gas without melting into a liquid first.
What is evaporation? - The process by which a liquid changes into a gas, usually caused by heat.
Yes, evaporation is the process by which a liquid changes into a gas, typically triggered by heat.
Explain the concept of kinetic energy in relation to states of matter. - Kinetic energy refers to the energy of motion. In solids, particles have the least kinetic energy, in liquids they have medium kinetic energy, and in gases, the particles have the highest kinetic energy.
Kinetic energy refers to the energy of motion. In terms of states of matter, particles in solids have the least kinetic energy as they vibrate in fixed positions, while particles in liquids have medium kinetic energy as they can move past each other but are still closely packed. In gases, particles have the highest kinetic energy as they move freely and collide with each other and the container walls at high speeds. Ultimately, the kinetic energy of particles in each state of matter influences their behavior and interactions with their surroundings.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments