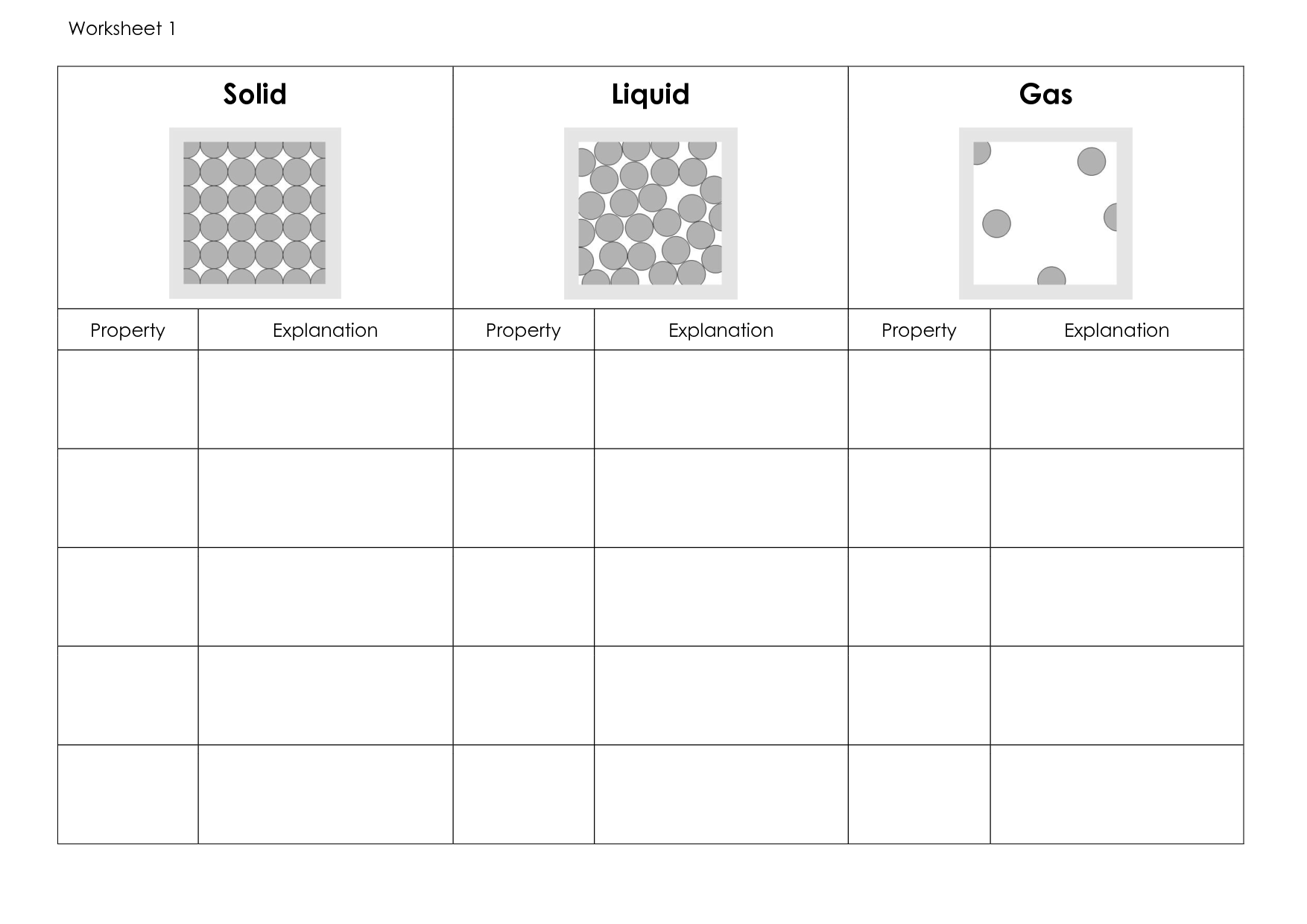
Solid-Liquid Gas Worksheet
Worksheets are an effective tool for educators looking to engage their students in a fun and interactive way. Whether you are a teacher seeking additional resources for your science curriculum or a parent hoping to supplement your child's learning at home, worksheets provide valuable opportunities to reinforce important concepts. One topic that often captures the curiosity of young learners is the study of solid, liquid, and gas states of matter. With a solid-liquid-gas worksheet, both educators and parents can effectively teach and assess their students' understanding of this fundamental scientific concept.
Table of Images 👆
- Solid Liquid and Gas Worksheets
- Solids Liquids and Gases Worksheets
- Solid-Liquid Gas Science Project
- Phase Diagram Worksheet
- Matter Solid-Liquid Gas Worksheet
- Physical Properties of Matter Worksheet
- Compare Solids Liquids and Gases
- Periodic Table Basics Worksheet Answer Key
- Separating Mixtures Worksheet
- Separating Mixtures Worksheet
- Separating Mixtures Worksheet
- Separating Mixtures Worksheet
- Separating Mixtures Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the state of matter in which particles are tightly packed together and have a definite shape?
The state of matter in which particles are tightly packed together and have a definite shape is a solid.
What is the state of matter in which particles are closely packed together but do not have a definite shape?
The state of matter in which particles are closely packed together but do not have a definite shape is a liquid. Liquids take the shape of their container due to the ability of their particles to move past each other while maintaining close proximity.
What is the state of matter in which particles are spread out and have no definite shape?
The state of matter in which particles are spread out and have no definite shape is called a gas. In this state, the particles are free to move around and occupy the volume of their container, taking on the shape of that container.
What happens to the particles when a solid is heated and reaches its melting point?
When a solid is heated and reaches its melting point, the particles within the solid start to gain enough energy to overcome the forces that hold them in a fixed, ordered arrangement. This causes the particles to break free from their fixed positions and start to move more freely, transitioning from a solid state to a liquid state. This process is known as melting, and it essentially involves a change from a rigid, ordered structure to a more disordered, fluid-like state as the particles gain enough energy to break their bonds and move more freely.
What happens to the particles when a liquid is cooled and reaches its freezing point?
When a liquid is cooled and reaches its freezing point, the particles in the liquid begin to lose energy, slow down, and come closer together. Eventually, the particles arrange themselves in a more orderly and structured manner, forming a solid. This process is known as freezing or solidification, where the liquid transitions into a solid state.
What is the process called when a substance changes directly from a solid to a gas without going through the liquid state?
The process called "sublimation" occurs when a substance changes directly from a solid to a gas without passing through the liquid state. This process involves the solid particles gaining enough energy to break free from the solid phase and transition into the gas phase without first becoming a liquid.
What is the process called when a substance changes directly from a gas to a solid without going through the liquid state?
The process is called deposition.
How does increasing pressure affect the temperature at which a substance changes from a liquid to a gas?
Increasing pressure typically increases the temperature at which a substance changes from a liquid to a gas. This is because higher pressure compresses the molecules closer together, requiring more thermal energy to overcome the intermolecular forces keeping them as a liquid. This is known as the Le Chatelier's principle, where applying pressure will push the equilibrium towards the state with fewer molecules (in this case, the gaseous state), requiring a higher temperature to achieve that equilibrium.
How does decreasing pressure affect the temperature at which a substance changes from a gas to a liquid?
Decreasing pressure lowers the temperature at which a substance changes from a gas to a liquid. This is because lower pressure reduces the kinetic energy required for particles to overcome the intermolecular forces holding them in the gas phase, allowing them to condense into a liquid at a lower temperature.
Give an example of a substance that can exist in all three states of matter at room temperature.
An example of a substance that can exist in all three states of matter at room temperature is water. At room temperature (around 20-25°C), water can be found as a solid (ice), liquid (water), and gas (water vapor).
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments