Practice Naming Acids Worksheet
Are you struggling to master the art of naming acids? Look no further. This practice naming acids worksheet is designed to help middle school students and high school chemistry enthusiasts understand the rules and nomenclature behind acid naming. By providing a variety of examples and exercises, this worksheet aims to crystalize the concepts surrounding acid names and make them easier to grasp.
Table of Images 👆
- Naming Acids Worksheet Answer Key
- Naming Binary Covalent Compounds Worksheet Answers
- Naming Acids and Bases Worksheet Answers
- Naming Covalent Compounds Worksheet
- Ionic Compounds Worksheet
- Naming Ionic Compounds Worksheet Answers
- Naming Acids and Bases Worksheet Answer Key
- Acids and Bases Worksheet
- Naming Chemical Compounds Worksheet Answers
- Acids and Bases Worksheet
- Naming Ionic and Covalent Compounds Worksheet
- Chemical Nomenclature Worksheet
- Naming Ionic Compounds Worksheet Answer Key
- Acids and Bases Worksheet Answers
- Naming Acids and Bases Worksheet Answers
- Alkane Alkene Alkyne Naming Worksheet with Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the purpose of a Practice Naming Acids Worksheet?
The purpose of a Practice Naming Acids Worksheet is to help students develop and reinforce their understanding of how to name different types of acids in chemistry. By completing the worksheet, students can practice identifying the formulas of acids and determining the correct naming conventions based on their chemical composition, which can improve their overall comprehension and retention of key concepts related to acids in chemistry.
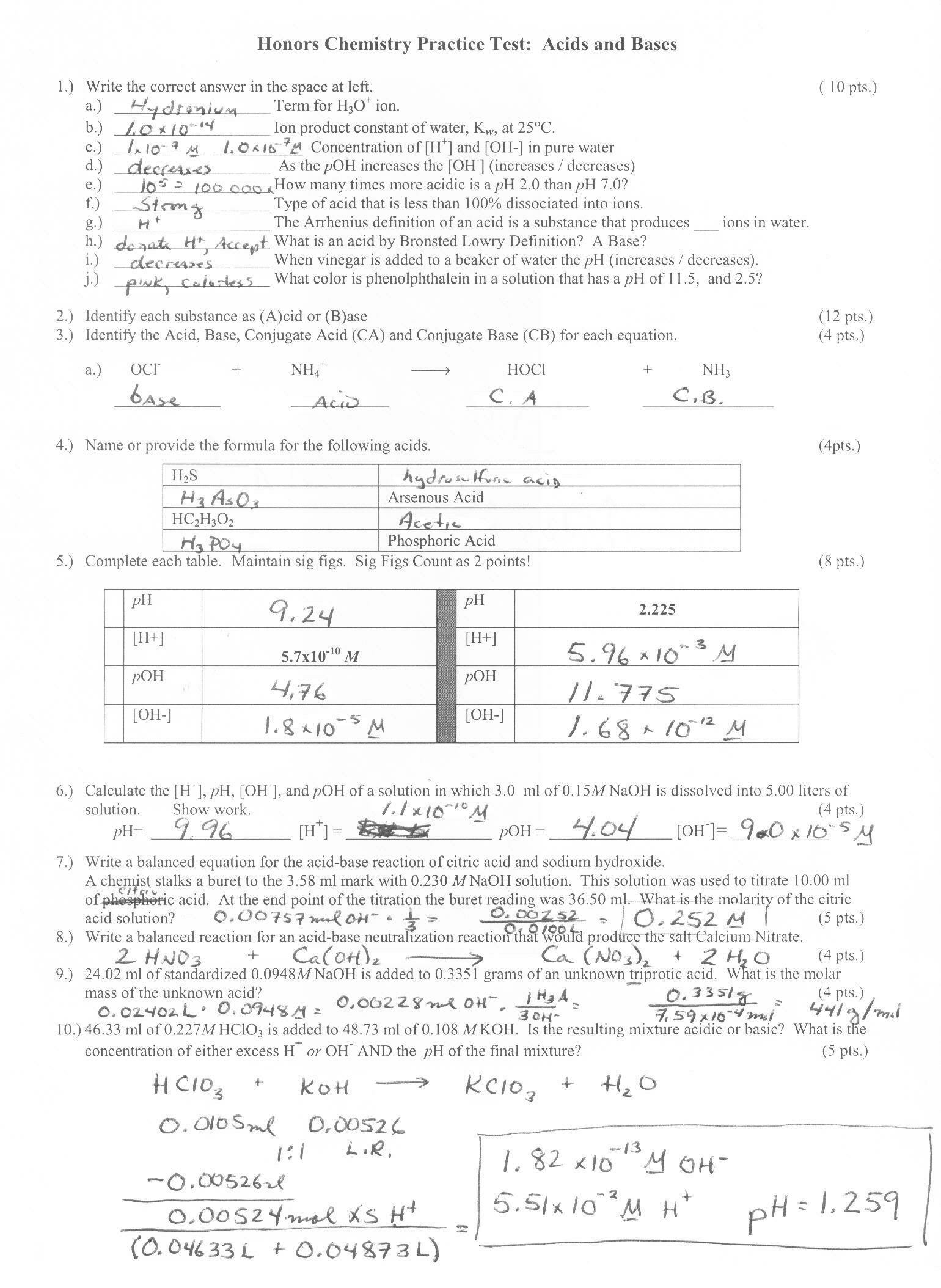
What is an acid?
An acid is a chemical compound that donates protons or hydrogen ions in a chemical reaction, resulting in a solution with a pH less than 7. Acids are known for their sour taste, ability to dissolve metals, and their role in chemical reactions such as neutralization and corrosion.
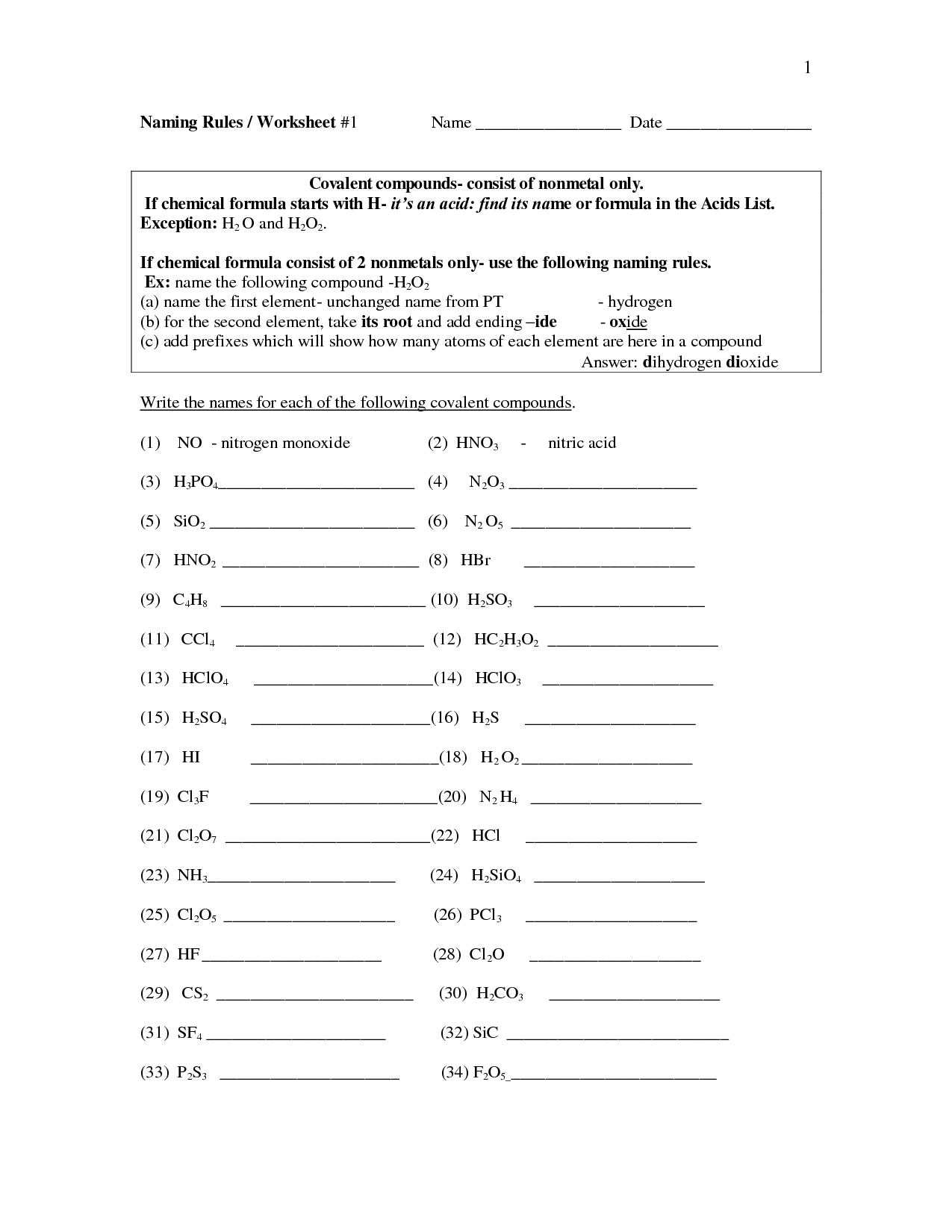
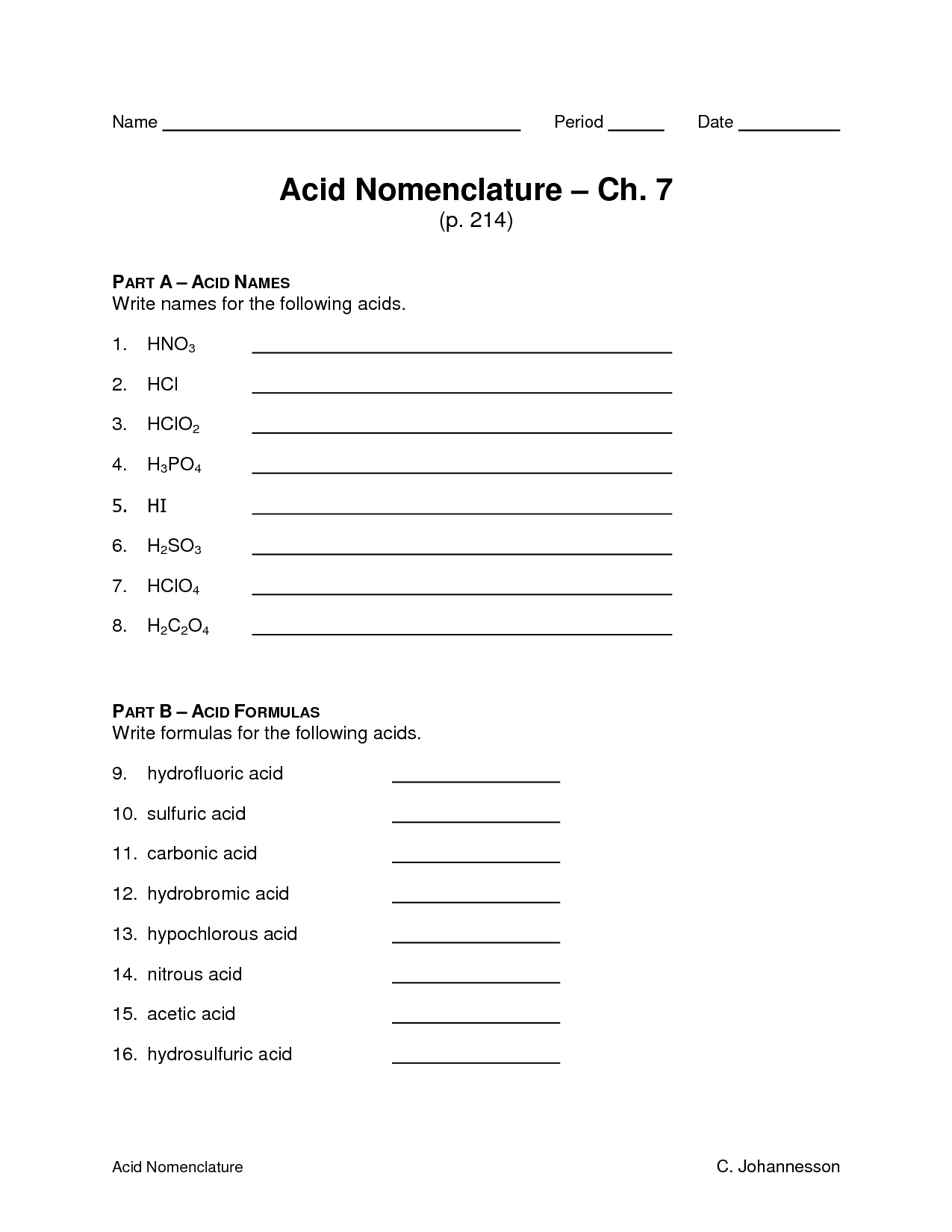
How do you determine the name of a binary acid?
To determine the name of a binary acid, you use the prefix "hydro-" followed by the root of the nonmetal element with the suffix "-ic." For example, HCl is hydrochloric acid, HBr is hydrobromic acid, and HI is hydroiodic acid.
How do you determine the name of a ternary acid?
To determine the name of a ternary acid, identify the polyatomic ion present in the compound. The polyatomic ion provides a clue to the acid name as follows: if the polyatomic ion ends in "-ate," the corresponding acid name ends in "-ic acid" (e.g., sulfate becomes sulfuric acid). If the polyatomic ion ends in "-ite," the corresponding acid name ends in "-ous acid" (e.g., sulfite becomes sulfurous acid). Remember the prefixes of the polyatomic ions as they guide the naming process.
What is the difference between a binary and a ternary acid?
The main difference between a binary acid and a ternary acid lies in their chemical composition. Binary acids consist of two elements - hydrogen and a nonmetal element, such as hydrochloric acid (HCl). Ternary acids, on the other hand, consist of three elements - hydrogen, a nonmetal element, and oxygen, such as sulfuric acid (H2SO4). This extra element in ternary acids gives them different properties and chemical behaviors compared to binary acids.
What is the role of hydrogen in an acid?
In an acid, hydrogen plays a crucial role by donating protons when dissolved in water, thereby increasing the concentration of H+ ions in the solution. This release of H+ ions is what gives acids their characteristic acidic properties, such as their ability to react with bases and form water. The strength of an acid is determined by its ability to donate hydrogen ions, with stronger acids donating more H+ ions than weaker acids.
How do you know the appropriate suffix to use when naming an acid?
To determine the appropriate suffix for naming an acid, you first need to identify the anion present in the acid. If the anion ends in "-ide," the corresponding acid will end in "-ic acid." If the anion ends in "-ite," the acid will end in "-ous acid." If the anion ends in "-ate," the acid will end in "-ic acid." By recognizing the type of anion present, you can apply the correct suffix to name the acid accordingly.
What are some common examples of binary acids?
Hydrochloric acid (HCl), hydrofluoric acid (HF), hydrobromic acid (HBr), and hydroiodic acid (HI) are common examples of binary acids, which are acids composed of only two elements - hydrogen and a halogen.
What are some common examples of ternary acids?
Common examples of ternary acids include sulfuric acid (H2SO4), phosphoric acid (H3PO4), nitric acid (HNO3), carbonic acid (H2CO3), and chloric acid (HClO3). These acids contain three different elements - hydrogen, oxygen, and one other element - and exhibit varying levels of acidity in aqueous solution.
Why is it important to practice naming acids?
It is important to practice naming acids because it helps strengthen one's understanding of their chemical composition and properties, which are crucial in various scientific fields such as chemistry, biology, and environmental science. By practicing naming acids, individuals can develop skills in nomenclature that are essential for proper communication and comprehension within the scientific community, enabling them to accurately discuss and interpret research findings, conduct experiments, and solve problems related to acid-base chemistry.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments