Periodic Table Puzzle Worksheet Answers
The Periodic Table Puzzle Worksheet provides an interactive and engaging way for chemistry enthusiasts to test their knowledge and understanding of elements' properties and characteristics. This worksheet is designed for students and learners who are interested in deepening their understanding of periodic table elements and how they interact within the chemical world.
Table of Images 👆
- Alien Periodic Table Worksheet Answers
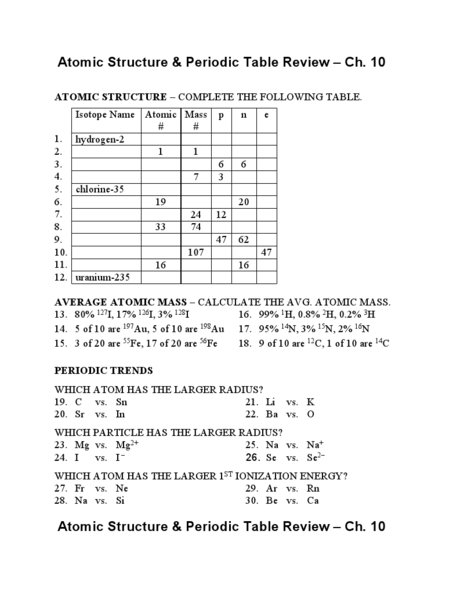
- Review Atomic Structure and Periodic Table
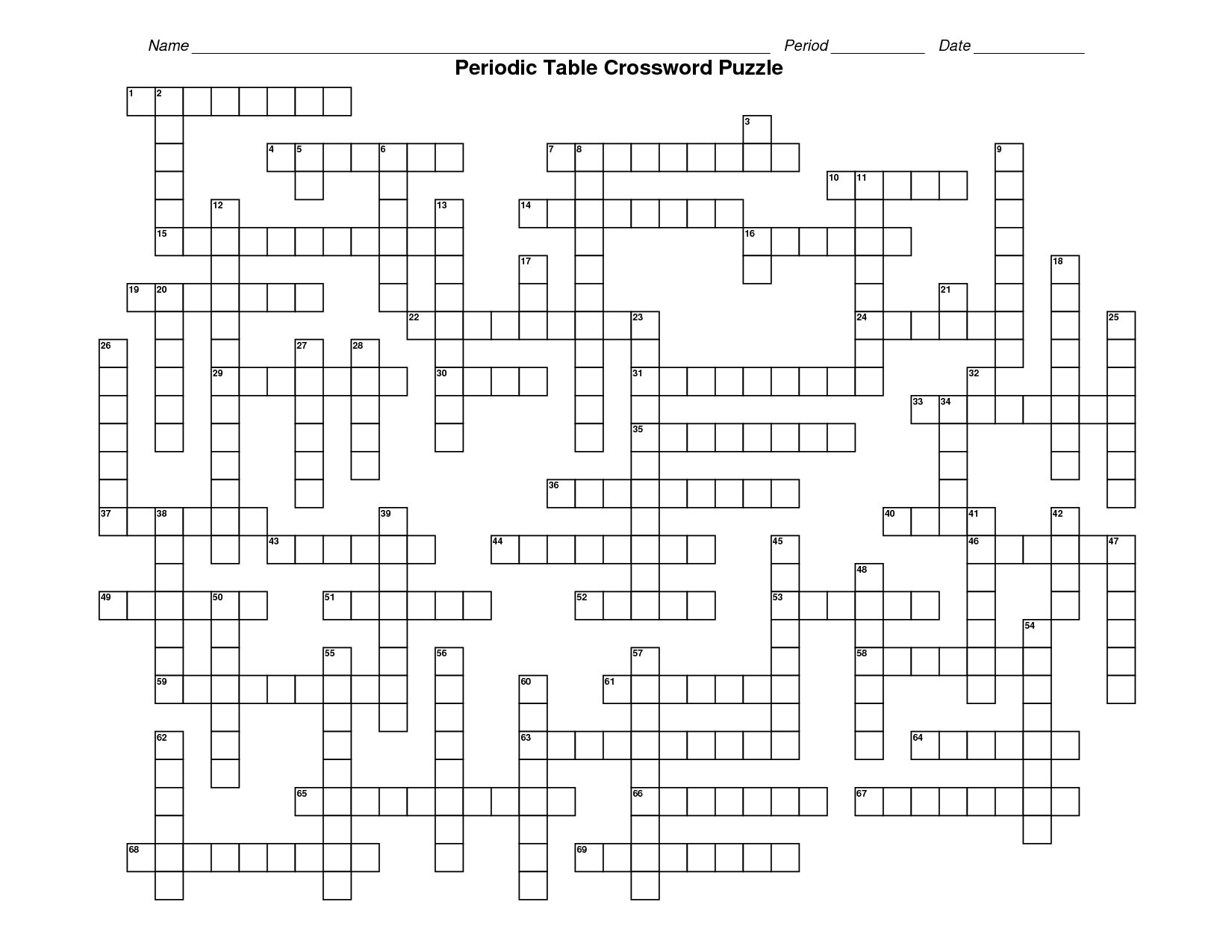
- Periodic Table Crossword Puzzle
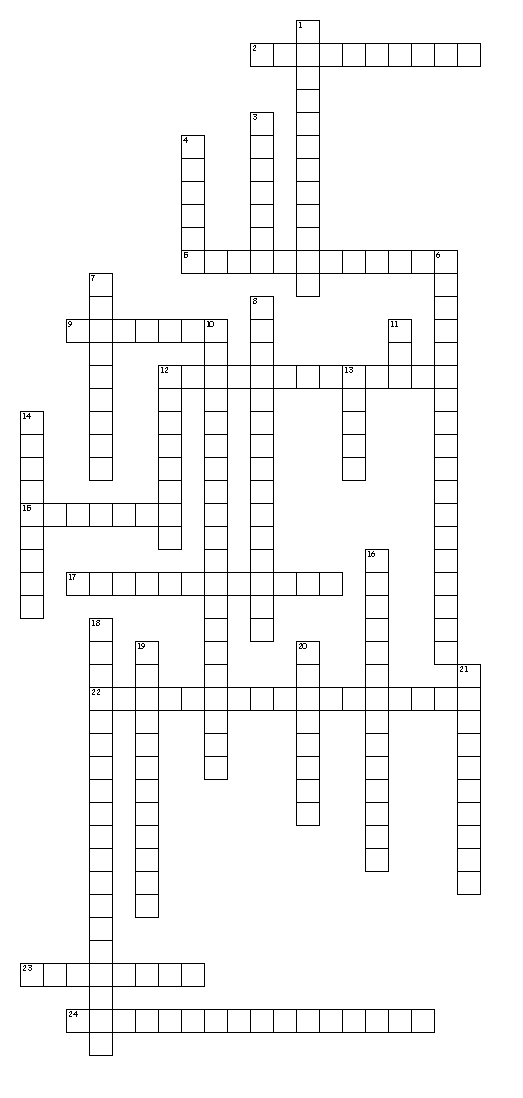
- Periodic Table Crossword Puzzle Answers
- Periodic Table Crossword Puzzle Answers
- Counting Atoms in Compounds Worksheet Answers
- Similar Triangles Practice Worksheet
- Periodic Table Puzzle Worksheet
- Chemistry Element Crossword Puzzle
- Organic Chemistry Crossword Puzzle Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is the atomic number of the element with the symbol "Na"?
The atomic number of the element with the symbol "Na" is 11.
Sodium (11)
Sodium (11) is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal that is part of the alkali metal group of the periodic table. Sodium is essential for various bodily functions in humans and is commonly found in table salt (sodium chloride). It is used in a wide range of applications, including in the production of sodium hydroxide, sodium carbonate, and as a component in various compounds and materials.
Which element has the highest atomic number?
The element with the highest atomic number is Oganesson, which has an atomic number of 118.
Oganesson (118)
Oganesson is a synthetic element with the atomic number 118, symbol Og, and atomic mass of approximately 294. It belongs to the noble gas group on the periodic table and is one of the heaviest known elements. Oganesson is highly unstable and radioactive, with a very short half-life, making it difficult to study its chemical properties.
What is the group number of the element with the symbol "Fe"?
The group number of the element with the symbol "Fe" is 8.
Iron (Group 8)
Iron is a metal in Group 8 of the periodic table, also known as the transition metals. It is a common element with the symbol Fe and atomic number 26. Iron is a crucial element for various biological processes as well as in industry due to its strength, durability, and magnetic properties. It is used in the production of steel, which is a vital material in construction, infrastructure, and manufacturing. Additionally, iron is an essential nutrient for the human body, playing a key role in the transportation of oxygen in the blood.
Which halogen has the lowest atomic number?
Fluorine has the lowest atomic number among the halogens, with an atomic number of 9.
Fluorine (9)
Fluorine is a chemical element with the atomic number 9, denoted by the symbol F. It is a highly reactive, pale yellow gas that is part of the halogen group on the periodic table. Fluorine is commonly found in compounds such as fluoride, which play essential roles in various biological processes and industrial applications.
What is the period number of the element with the symbol "Cu"?
The period number of the element with the symbol "Cu" is 4.
Copper (Period 4)
Copper is a chemical element with the symbol Cu and atomic number 29. It is a transition metal and belongs to period 4 of the periodic table. Copper is known for its excellent conductivity of heat and electricity, making it a crucial material in various industries, such as electronics and construction. Additionally, copper is highly malleable and ductile, allowing it to be easily shaped into various forms.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments