Osmosis Worksheet Answers
Worksheets are valuable tools that enable students to practice and reinforce their understanding of various subjects. For those seeking a comprehensive resource to test their knowledge on the process of osmosis, look no further than Osmosis Worksheet Answers. This carefully crafted set of questions and solutions provides a targeted and effective way for biology students to grasp the concept of osmosis and its related principles.
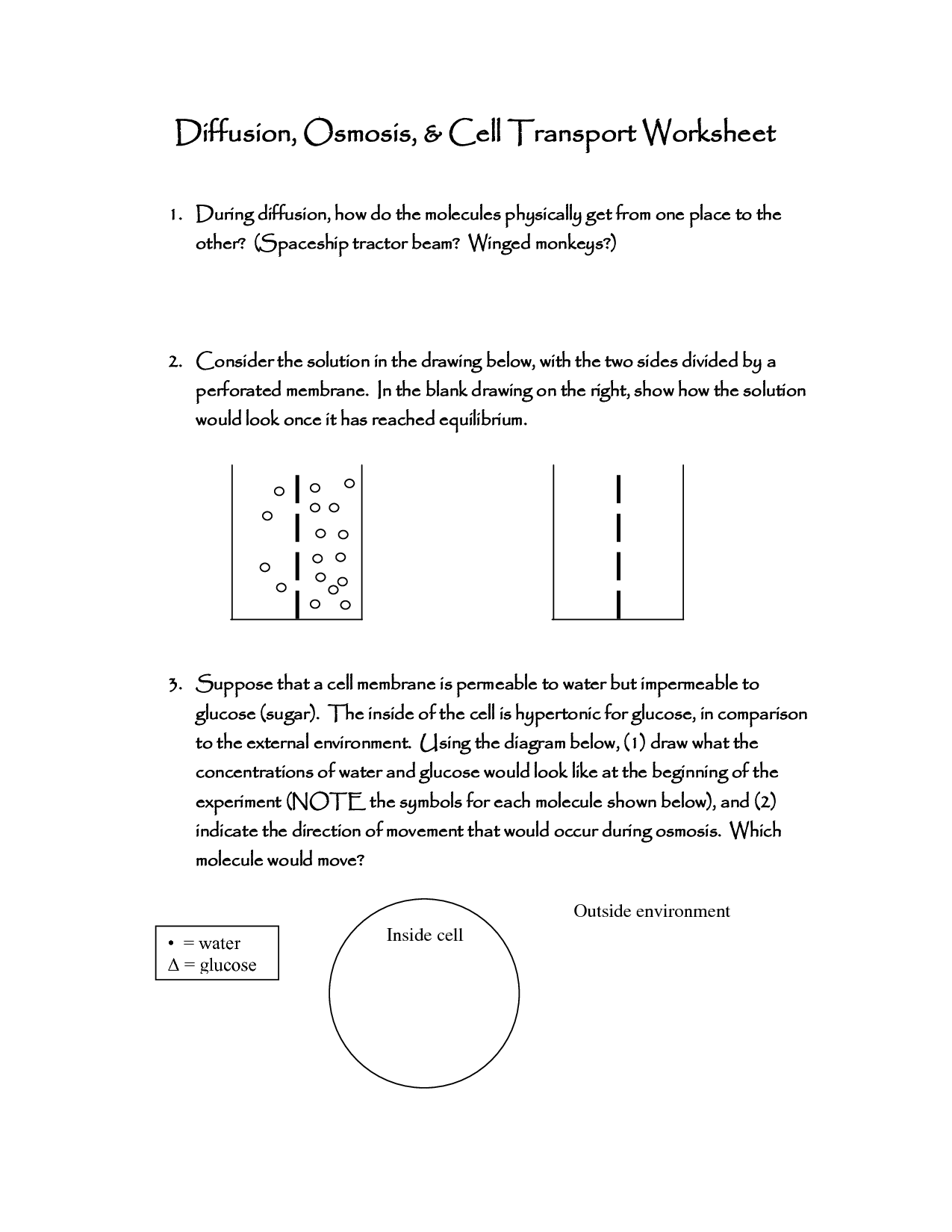
Table of Images 👆
- Osmosis and Tonicity Worksheet Answer Key
- Osmosis Jones Worksheet Answer Key
- Osmosis Jones Worksheet Answer Key
- Osmosis Jones Worksheet Answers
- Osmosis Jones Worksheet Answer Key
- Diffusion and Osmosis Worksheet Answer Key
- Osmosis Jones Worksheet Answer Key
- Osmosis Jones Worksheet Answers
- Diffusion and Osmosis Worksheet Answers
- Diffusion and Osmosis Worksheet Answers
- Osmosis Jones Biology Worksheet Answers
- Osmosis Jones Worksheet Answers
- Osmosis Jones Worksheet Answer Key
- Diffusion and Osmosis Worksheet Answers
- Cell Transport Diffusion and Osmosis Worksheet
- Osmosis Jones Movie Worksheet Answers
- Diffusion and Osmosis Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is osmosis?
Osmosis is the process by which molecules of a solvent, typically water, pass through a semipermeable membrane from a less concentrated solution into a more concentrated solution, equalizing the concentrations on both sides of the membrane. This movement of water is driven by the concentration gradient, with water moving from an area of lower solute concentration to an area of higher solute concentration, in an attempt to achieve equilibrium.
How does osmosis occur in a cell?
Osmosis occurs in a cell when there is a movement of solvent molecules, such as water, across a selectively permeable membrane from an area of higher water concentration to an area of lower water concentration. This movement is driven by the concentration gradient of solute molecules that cannot pass through the membrane, resulting in the equalization of solute concentrations on both sides of the membrane. Ultimately, osmosis plays a crucial role in maintaining the proper balance of water and solutes inside a cell to ensure its survival and proper functioning.
What is the role of a selectively permeable membrane in osmosis?
A selectively permeable membrane plays a critical role in osmosis by allowing the passage of certain molecules, typically water, while restricting the flow of others based on their size, charge, or chemical properties. This selective process enables the regulation of the movement of solutes and solvent across the membrane, thereby maintaining the balance of fluids and ions inside and outside the cell. In osmosis, water moves through the selectively permeable membrane from an area of higher water concentration to an area of lower water concentration, in response to the concentration gradient of solutes, ultimately helping to equalize the solute concentrations on both sides of the membrane.
What is the concentration gradient in osmosis?
The concentration gradient in osmosis is the difference in solute concentration between two solutions separated by a semi-permeable membrane. This gradient drives the movement of solvent molecules, typically water, from an area of lower solute concentration to an area of higher solute concentration, in order to establish equilibrium between the two solutions.
How does osmosis differ from diffusion?
Osmosis is a specific type of diffusion that involves the movement of water molecules across a semi-permeable membrane from an area of higher water concentration to an area of lower water concentration. Diffusion, on the other hand, refers to the overall movement of molecules from an area of higher concentration to an area of lower concentration, without the requirement of a semi-permeable membrane.
What are the three possible outcomes when a cell is placed in a hypotonic solution?
When a cell is placed in a hypotonic solution, three possible outcomes include: swelling or expanding due to water entering the cell to reach equilibrium with the higher concentration outside; bursting or lysing if the cell cannot regulate the influx of water and reaches its maximum capacity; and ultimately, if the cell is able to adapt and maintain osmotic balance, it will reach a state of turgor pressure where the cell membrane pushes against the cell wall to prevent bursting.
What happens to a cell in a hypertonic solution?
When a cell is placed in a hypertonic solution, which has a higher solute concentration than the inside of the cell, water will move out of the cell through osmosis. This causes the cell to shrink and potentially become dehydrated due to the loss of water. Ultimately, the cell may undergo a process called crenation, where it becomes wrinkled and distorted in shape due to the outward movement of water.
How does osmosis affect the size and shape of plant cells?
Osmosis affects the size and shape of plant cells by regulating the movement of water across their cell membranes. When plant cells are in a hypotonic environment (lower solute concentration outside the cell), water diffuses into the cell, causing it to swell and potentially burst. Conversely, in a hypertonic environment (higher solute concentration outside the cell), water moves out of the cell, leading to shrinkage and potential wilting. Osmosis plays a crucial role in maintaining turgor pressure within plant cells, which in turn influences their size, shape, and overall health.
How does osmosis contribute to the transport of water in plants?
Osmosis plays a crucial role in the transport of water in plants by allowing the movement of water molecules across a selectively permeable membrane. In plants, water from the soil travels through the roots and into the vascular system, where it is transported to various parts of the plant for photosynthesis, growth, and other essential processes. Osmosis helps maintain the water potential gradient needed for water uptake in plant cells, ensuring proper hydration and functioning of the plant tissues.
How does osmosis impact the ability of cells to maintain homeostasis?
Osmosis plays a crucial role in helping cells maintain homeostasis by regulating the movement of water and solutes across the cell membrane. Through osmosis, cells can control their internal environment by either taking in or releasing water to ensure the proper balance of ions and nutrients inside the cell. This process allows cells to maintain the right osmotic pressure, which is necessary for their proper functioning and to prevent damage from either swelling or shrinking due to changes in the surrounding environment. Ultimately, osmosis is essential for cells to maintain a stable internal environment and carry out their normal physiological functions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments