Organic Chemistry Worksheets
Organic chemistry worksheets provide a valuable tool for students to reinforce their understanding of key concepts and practice problem-solving skills. Designed to cater specifically to the needs of organic chemistry students, these worksheets offer a variety of exercises and questions that focus on essential topics and themes within the subject. Whether you're a student seeking additional practice or an instructor looking for supplemental materials, organic chemistry worksheets are a valuable resource to enhance your learning experience.
Table of Images 👆
- Chemistry Organic Compounds Worksheets
- Organic Chemistry Worksheets Answers
- Chemistry Vocabulary Worksheet
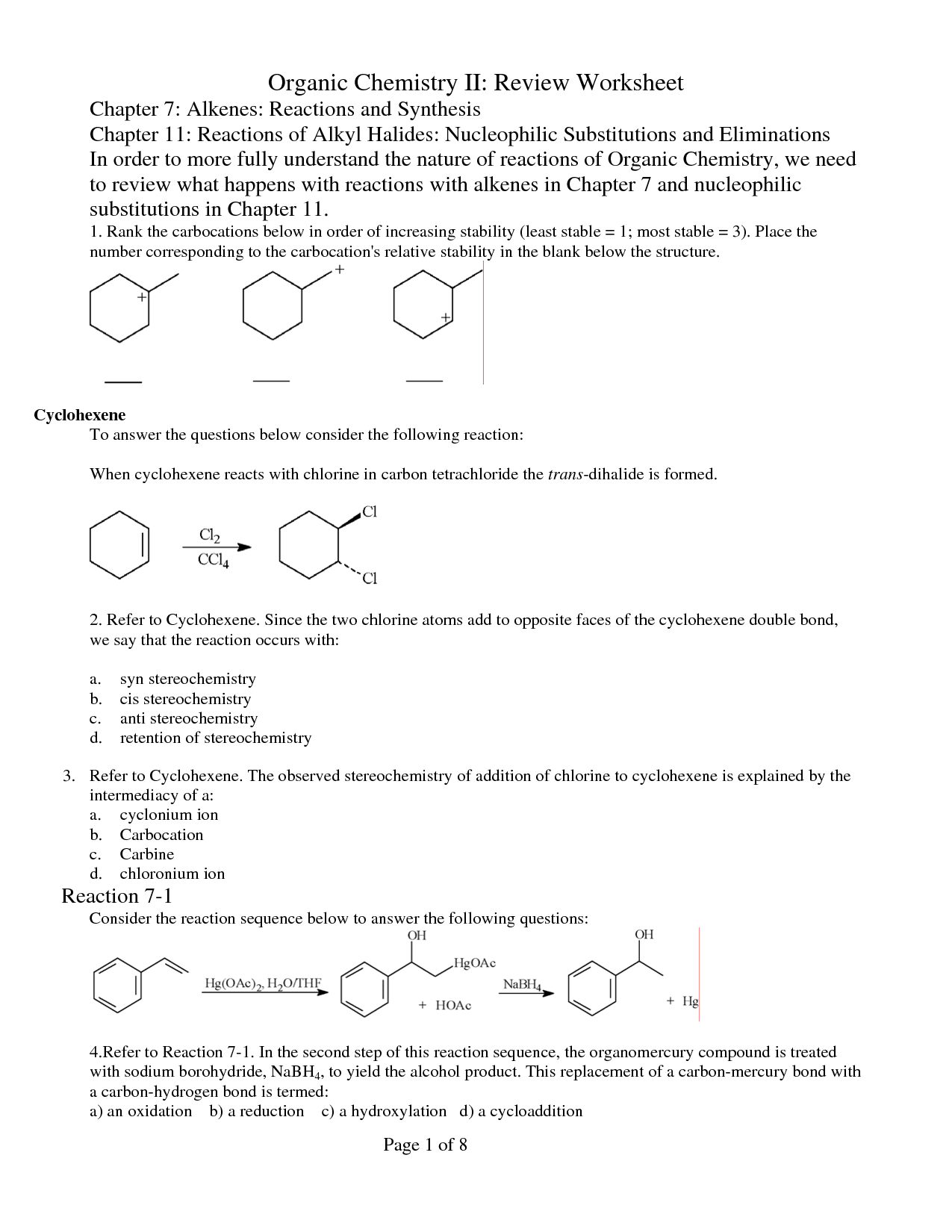
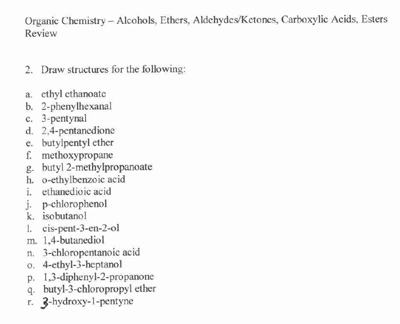
- Organic Chemistry Review Worksheets
- Alcohol Organic Chemistry Worksheet
- Organic Functional Groups Worksheet
- Organic Molecules Worksheet Review Answers
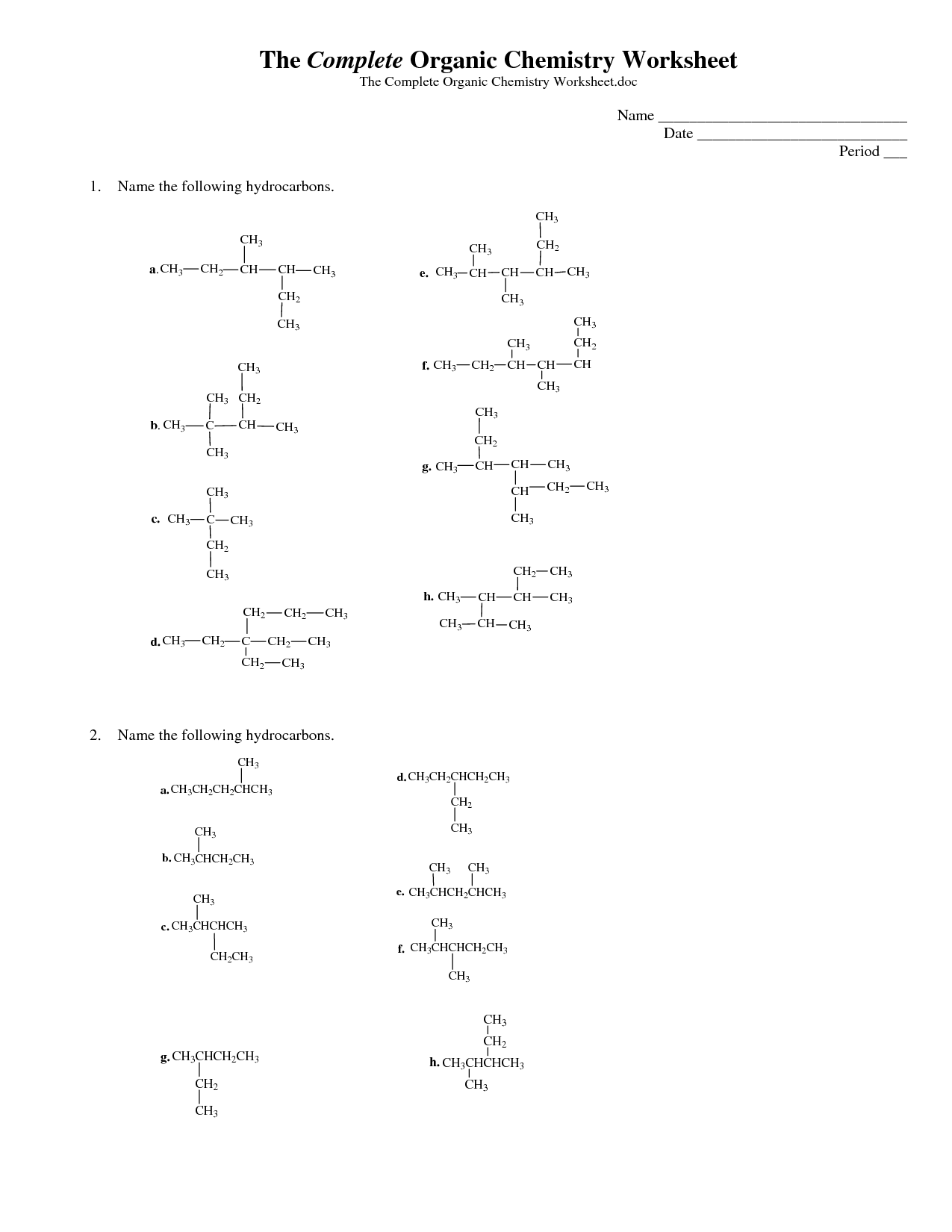
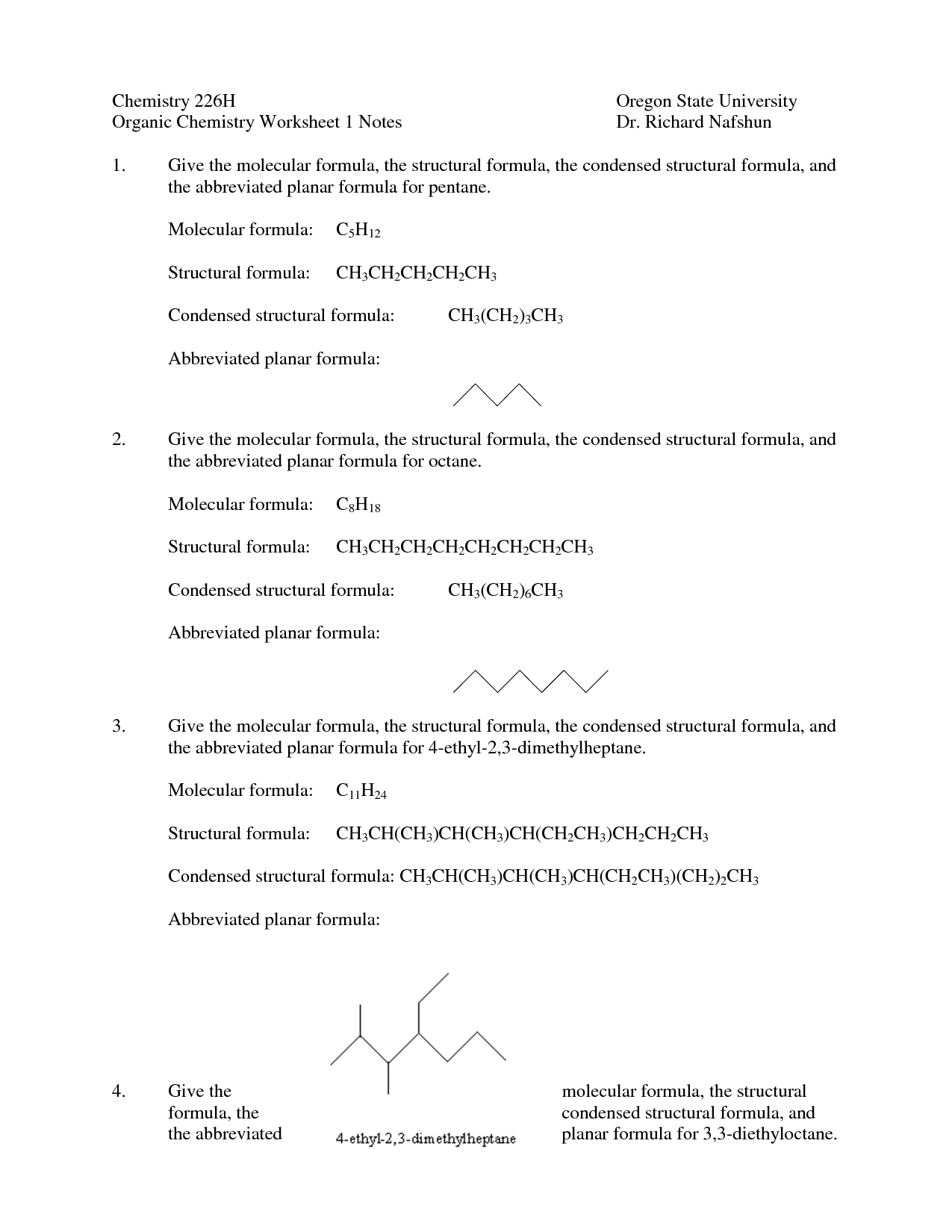
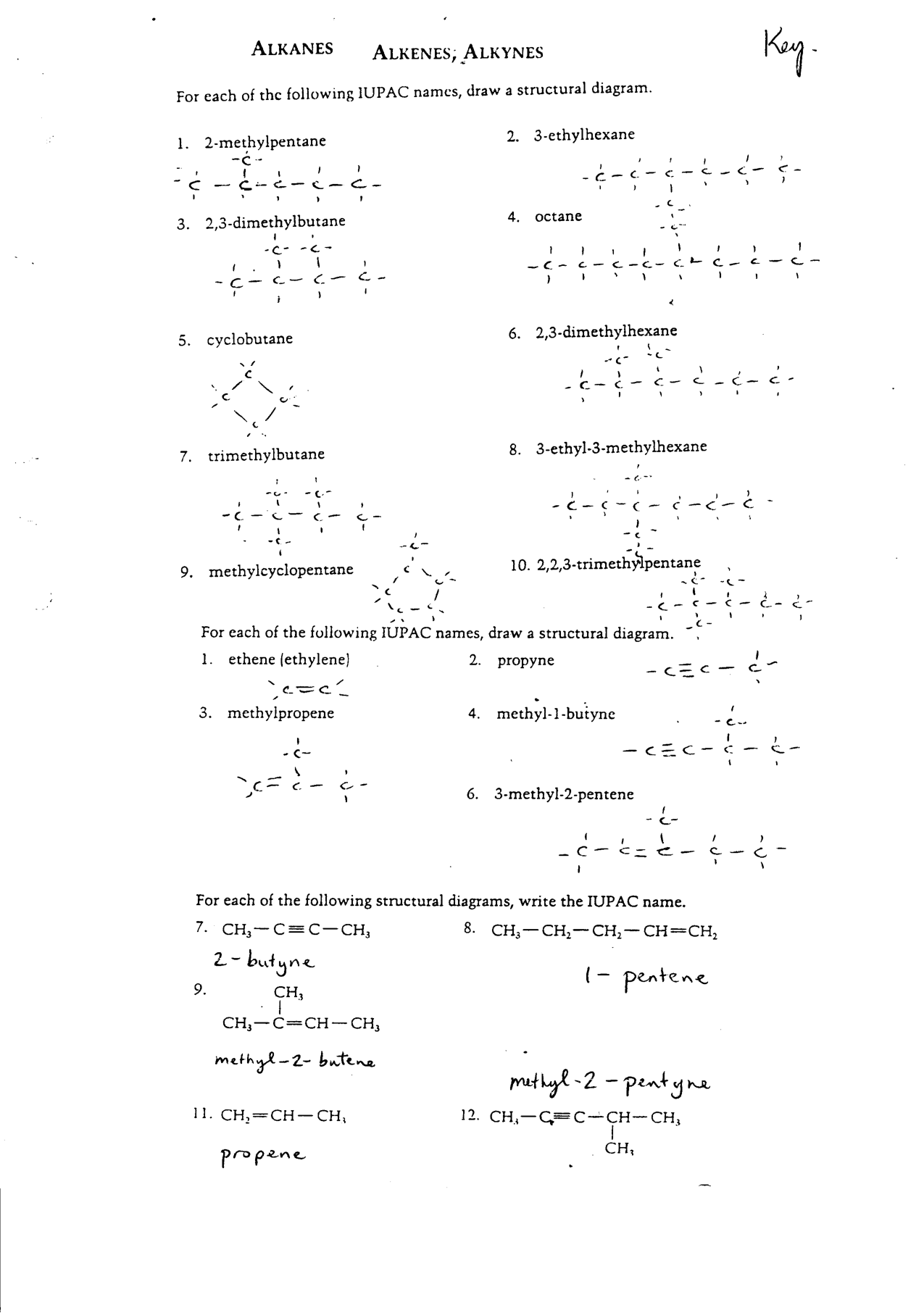
- Organic Chemistry Nomenclature Worksheet
- Naming Organic Compounds Worksheet
- Organic Compounds Structure Worksheet
- Organic Chemistry Nomenclature Worksheet
- Organic Chemistry Worksheets Answers
- Organic Chemistry Functional Groups Worksheet
- Organic Chemistry Nomenclature Worksheets with Answers
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
Explain the concept of hybridization in organic chemistry.
Hybridization in organic chemistry is a concept that involves the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. This process occurs when atoms bond to form molecules and is crucial for understanding the geometry and bonding behavior of molecules. By hybridizing atomic orbitals, such as s, p, and d orbitals, the resulting hybridized orbitals adopt specific geometries, such as sp, sp2, and sp3, which determine the overall molecular structure and properties. Hybridization helps explain bonding patterns, molecular shapes, and the overall stability of organic compounds.
Describe the mechanism for the electrophilic addition reaction of alkenes.
The electrophilic addition reaction of alkenes involves the π electrons of the double bond acting as nucleophiles attacking an electrophilic species, causing the double bond to break and creating two new sigma bonds. Initially, the electrophile approaches the π bond, forming a cyclic intermediate known as a carbocation. The carbocation is then attacked by the nucleophile, which results in the formation of two new sigma bonds. This process involves the rearrangement of electron density within the molecule, ultimately leading to the addition of atoms or groups across the double bond in order to satisfy the valency requirements.
Provide a detailed explanation of the formation of carbocations during organic reactions.
Carbocations form during organic reactions when a carbon atom loses an electron and becomes electron-deficient, leading to a positively charged carbon center. This electron deficiency can result from the breaking of a covalent bond between a carbon and another atom, leaving the carbon with only three bonds and a vacant p orbital. The stability of carbocations is influenced by factors such as resonance, inductive effects, and hyperconjugation. Resonance stabilization occurs when the positive charge can be delocalized across multiple atoms through pi bonds, increasing the stability of the carbocation. Inductive effects involve the transmission of electron density through sigma bonds, with electronegative atoms withdrawing electron density and destabilizing the carbocation. Hyperconjugation involves the overlap of a vacant p orbital with adjacent sigma bonds, providing additional stability by spreading out the positive charge. Overall, the formation and stability of carbocations play a crucial role in determining the outcome of organic reactions.
Discuss the concept of resonance in organic molecules and its significance.
Resonance in organic molecules is a theoretical concept where electrons are not restricted to a single bond but can delocalize over multiple atoms, leading to the stabilization of the molecule. This phenomenon helps explain the stability, reactivity, and structure of many organic compounds. By redistributing the electrons, resonance can impact the acidity, basicity, and overall chemical behavior of a molecule, influencing its properties and reactions. Understanding resonance is vital in predicting and explaining the behavior of organic molecules, guiding synthetic strategies, and analyzing reaction mechanisms in organic chemistry.
Explain the factors that influence the acidity of organic compounds.
The acidity of organic compounds is influenced by factors such as the stability of the conjugate base, the polarity of the bond between the acidic hydrogen and the rest of the molecule, and the resonance stabilization of the resulting anion. Electronegative atoms nearby, such as oxygen or halogens, can also increase acidity by stabilizing the resulting negative charge. Additionally, the presence of electron-withdrawing groups can increase acidity by pulling electron density away from the acidic hydrogen, making it easier for it to ionize. Furthermore, the size and inductive effects of substituents can affect the acidity of organic compounds.
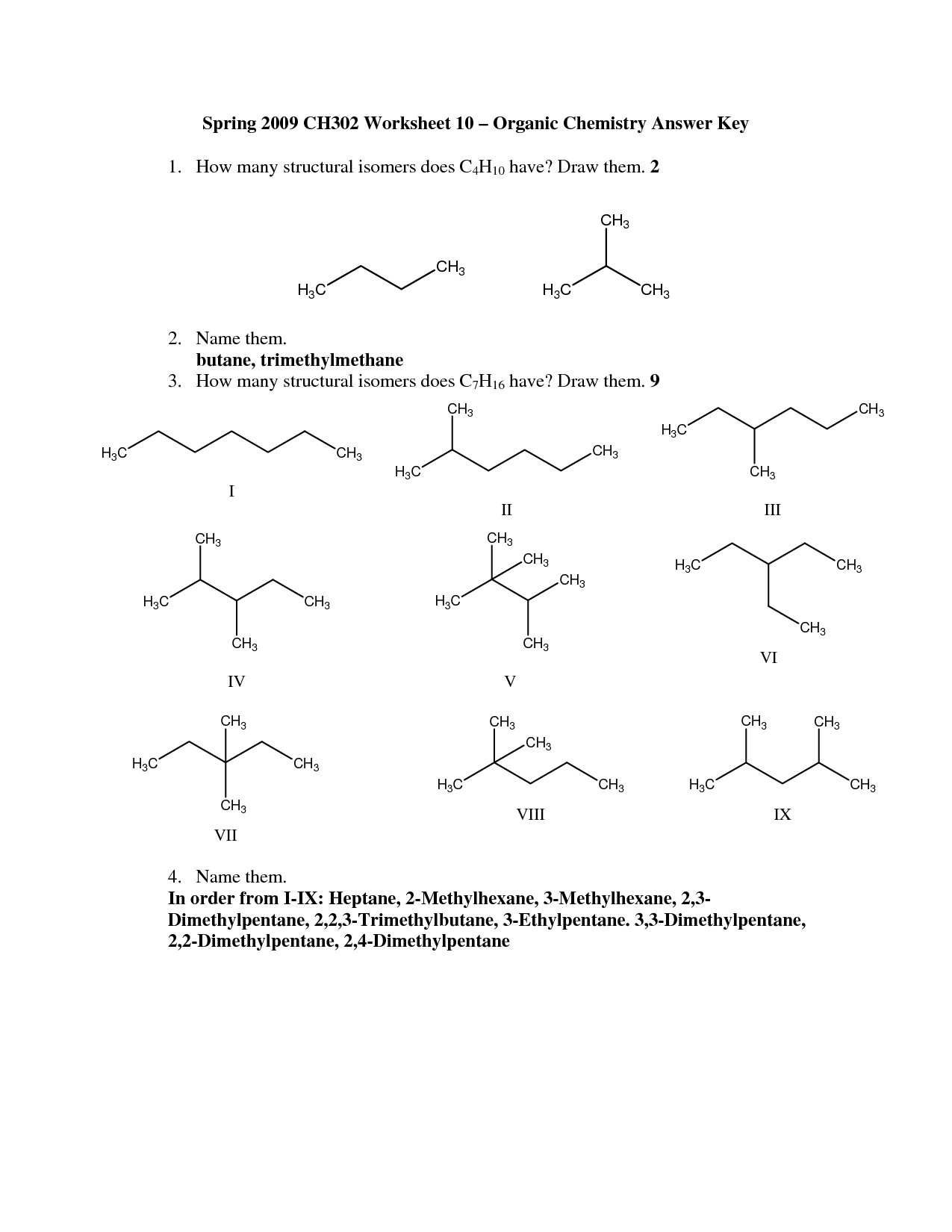
Describe the different types of isomerism found in organic compounds.
Organic compounds can exhibit different types of isomerism, including structural isomerism (where isomers differ in connectivity or arrangement of atoms), stereoisomerism (where isomers have the same connectivity but differ in spatial arrangement of atoms), and tautomeric isomerism (where isomers exist in equilibrium due to the migration of a hydrogen atom). Examples of stereoisomerism include geometric isomerism (cis-trans isomerism) and optical isomerism (enantiomers and diastereomers). Structural isomerism includes chain isomerism, position isomerism, and functional group isomerism, among others, showcasing the rich diversity of isomeric forms that organic compounds can take.
Discuss the principle of chirality and its importance in organic chemistry.
Chirality in organic chemistry refers to the property of molecules that are non-superimposable mirror images of each other, known as enantiomers. This property arises from the presence of a stereogenic center, typically a carbon atom with four different groups attached to it. Chirality plays a crucial role in organic chemistry as it determines the biological activity, pharmacological properties, and reactivity of molecules. Enantiomers exhibit different interactions with biological receptors, enzymes, and other molecules, leading to distinct physiological effects. Therefore, understanding and controlling chirality are essential in drug design, synthesis of complex natural products, and study of biochemical processes.
Explain the process of aromaticity and its significance in organic compounds.
Aromaticity is a property of some cyclic organic compounds where a ring of conjugated pi electrons follows Huckel's rule, which states that a molecule is aromatic if it has 4n+2 pi electrons in a planar, cyclic system. Aromatic compounds are known for exceptional stability and unique chemical properties. They often undergo substitution reactions rather than addition reactions, are less reactive towards oxidation and reduction, and exhibit resonance stabilization. Aromatic compounds are widely found in nature and are important in the field of organic chemistry due to their diverse applications in pharmaceuticals, fragrances, and materials science.
Provide a detailed explanation of the functional groups and their role in organic chemistry.
Functional groups are specific groups of atoms within a molecule that determine its chemical properties and reactivity. They are responsible for giving organic molecules their distinct characteristics and functionality. Each functional group has its own unique structure and behavior, allowing organic compounds to form a wide variety of molecules with different properties. Examples of functional groups include hydroxyl (-OH), carboxyl (-COOH), amino (-NH2), and carbonyl (-C=O). Depending on the functional group present, organic molecules can exhibit properties such as acidity, basicity, solubility, and stability, as well as participate in specific chemical reactions. Understanding functional groups is crucial in organic chemistry as it helps predict how molecules will interact and behave in various chemical reactions and biological processes.
Discuss the concept of nucleophilic substitution reactions and provide examples.
Nucleophilic substitution reactions involve the substitution of a leaving group on a molecule with a nucleophile, resulting in a new compound. These reactions are common in organic chemistry and can occur through either SN1 or SN2 mechanisms. In SN1 reactions, the leaving group first dissociates, forming a carbocation intermediate that is then attacked by a nucleophile. On the other hand, SN2 reactions involve a one-step process in which the nucleophile attacks the substrate at the same time as the leaving group departs. Examples of nucleophilic substitution reactions include the hydrolysis of alkyl halides by water, as well as the reaction of primary alkyl halides with sodium cyanide to form nitriles.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments