Organic Chemistry Naming Worksheet
Are you studying organic chemistry and struggling with the complex task of naming compounds? Look no further than our organic chemistry naming worksheet. Designed specifically for students like you, this worksheet provides a comprehensive, structured approach to mastering the art of organic compound naming. Whether you're a beginner or just looking for some extra practice, this worksheet will help you grasp the essential concepts and become more confident in your understanding of organic chemistry naming.
Table of Images 👆
- Straight Chain Alkanes
- Alkane Nomenclature Worksheet with Answers
- Organic Chemistry Nomenclature Worksheet
- Organic Chemistry Worksheets
- Naming Organic Compounds Worksheet
- Practice Balancing Chemical Equations Worksheet
- Naming Ionic Compounds Worksheet Answer Key
- Organic Chemistry Structural Formulas
- Drawing Hydrocarbons Worksheet
- Organic Chemistry Naming Alkanes
- Chemistry Stoichiometry Worksheet Answer Key
- Naming Alkanes Alkenes Alkynes Worksheet
- Chemical Formula for Polyatomic Ions
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the purpose of organic chemistry naming?
The purpose of organic chemistry naming is to systematically and uniquely identify organic compounds based on their structure and composition. This naming system allows chemists to communicate effectively, establish a standardized way to refer to compounds, and convey important information about their molecular structure, functional groups, and properties. By following specific rules and conventions, organic chemistry naming enables accurate identification, classification, and communication of the vast array of organic compounds found in nature and synthesized in laboratories.
What are functional groups in organic chemistry?
Functional groups in organic chemistry are specific arrangements of atoms or bonds within molecules that exhibit characteristic chemical properties and reactions. These groups can significantly influence the reactivity, polarity, and physical properties of organic compounds. Some common functional groups include hydroxyl (-OH), carbonyl (C=O), amino (-NH2), and carboxyl (-COOH) groups, each imparting distinct chemical behavior to the molecules they are a part of.
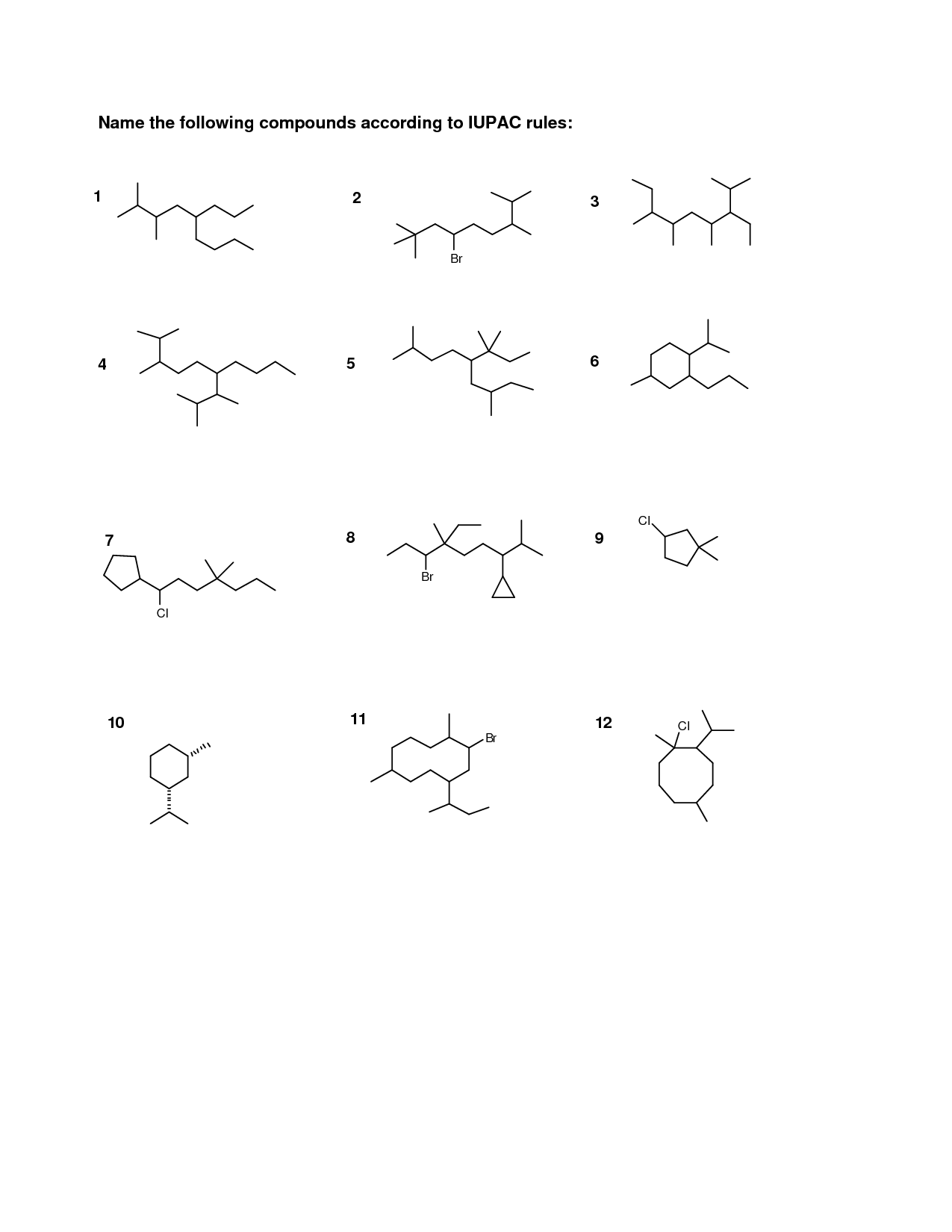
How are alkanes named?
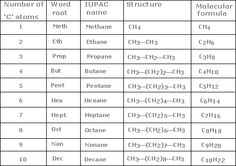
Alkanes are named based on the number of carbon atoms in the longest continuous chain within the molecule. The name typically begins with the prefix indicating the number of carbon atoms in the chain (meth- for 1 carbon, eth- for 2 carbons, prop- for 3 carbons, but- for 4 carbons, etc.) followed by the suffix -ane to indicate that the molecule is an alkane. Additionally, any branches or substituents on the carbon chain are named using prefixes like methyl, ethyl, propyl, etc., and their positions are indicated by numbering the carbon atoms in the main chain.
How are alkenes and alkynes named?
Alkenes and alkynes are named by identifying the longest carbon chain that contains the double or triple bond respectively, and then numbering the carbon atoms in the chain to give the double or triple bond the lowest possible number. The alkene suffix is "-ene", while the alkyne suffix is "-yne". Additionally, any substituents attached to the carbon chain are named using the appropriate prefixes and placed in front of the parent chain name.
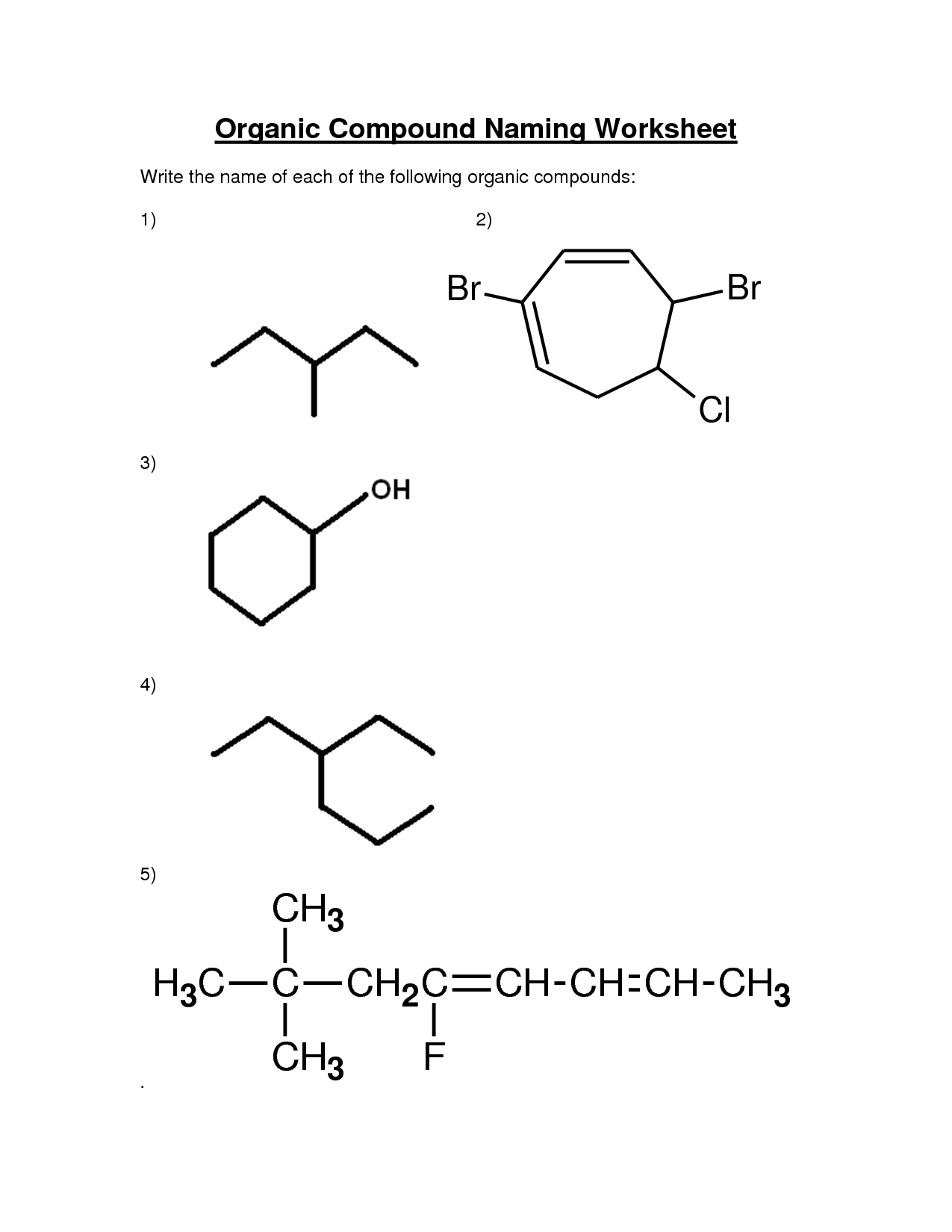
What is the naming convention for alcohols?
Alcohols are named by adding the suffix "-ol" to the root name of the parent hydrocarbon, with the position of the hydroxyl group indicated by a number if necessary. The hydroxyl group takes precedence over other functional groups when naming the compound, and the carbon atom to which it is bonded is assigned the lowest possible number.
How do you name aldehydes and ketones?
Aldehydes are named by replacing the "-e" ending of the parent alkane with "-al", while ketones are named by replacing the "-e" in the parent alkane with "-one". The carbon chain is numbered starting from the end closest to the functional group, and the location of the carbonyl group is indicated by a number. In cases of multiple functional groups or substituents, these are designated by prefixes such as "di-" or "tri-".
What are carboxylic acids and how are they named?
Carboxylic acids are organic compounds that contain a carboxyl group, which consists of a carbonyl group (C=O) and a hydroxyl group (?OH) attached to the same carbon atom. They are named by replacing the -e at the end of the parent alkane name with -oic acid. The carbon chain is numbered to give the carboxyl carbon the lowest possible number, and any substituents are indicated by their positions on the chain and named alphabetically.
How are esters named in organic chemistry?
Esters are named by taking the alkyl or aryl group from the alcohol and the alkyl or aryl group from the carboxylic acid, and combining them in that order along with the suffix "-oate." The alcohol group is named first, followed by the carboxylic acid group with the "-oate" ending. For example, the ester formed from ethanol and acetic acid is named ethyl acetate.
What are amines and how are they named?
Amines are organic compounds that contain a nitrogen atom bonded to hydrogen atoms or carbon groups. They are named by replacing the "-e" in the corresponding alkane name with "-amine." The carbon chain to which the nitrogen is attached is numbered to locate the amine group, and any alkyl substituents are labeled using the N- prefix to indicate their attachment to the nitrogen atom. The naming of amines can also include prefixes like di-, tri-, or N,N-dimethyl- to indicate multiple substituents.
What is the significance of organic chemistry naming in the field of chemical synthesis?
Organic chemistry naming is crucial in the field of chemical synthesis as it allows chemists to accurately communicate the structures of the compounds they are working with. This systematic nomenclature helps in identifying different functional groups, determining reactivity, predicting reaction outcomes, and designing synthesis routes. By understanding and using organic chemistry naming conventions, chemists can efficiently plan and execute complex synthesis processes to create new molecules with specific properties and functions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments