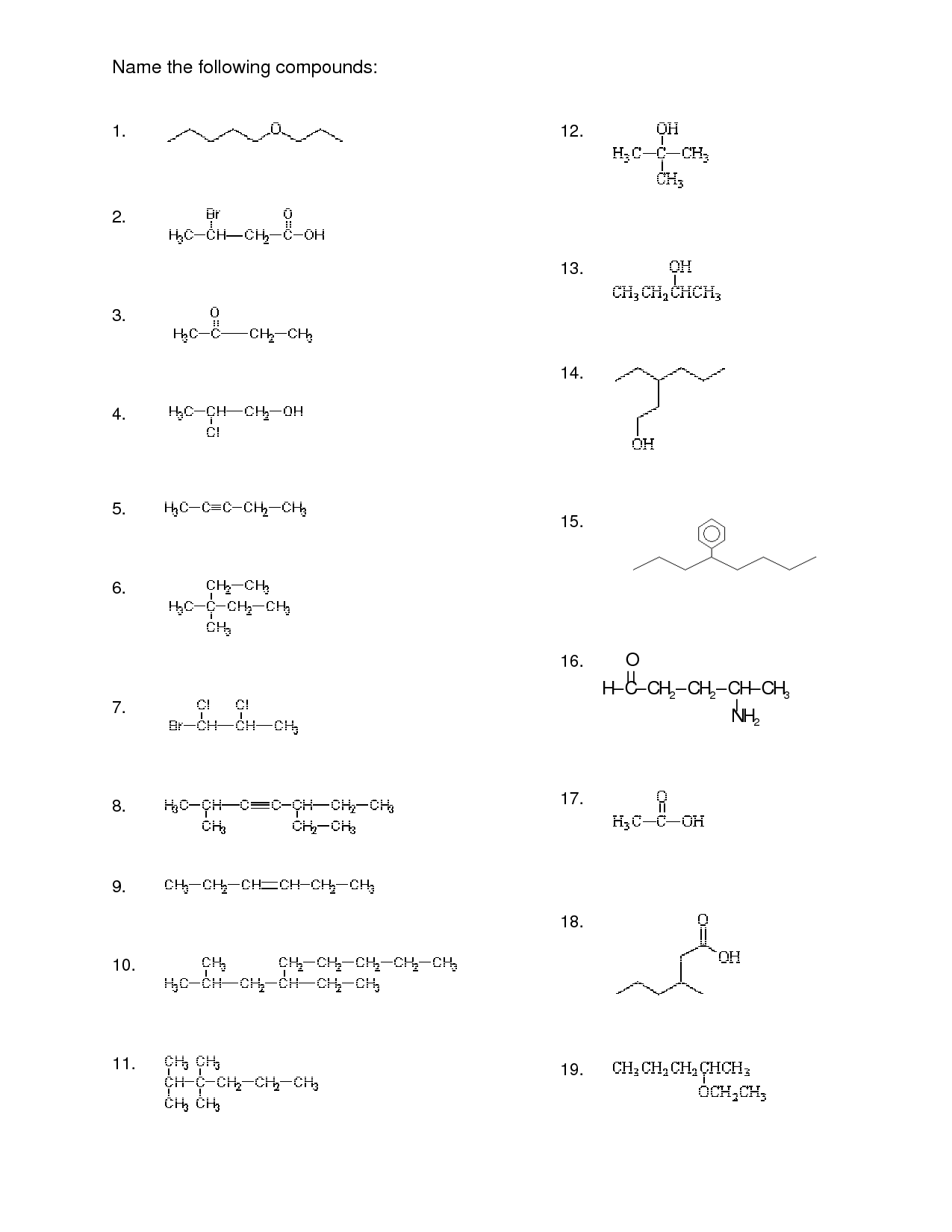
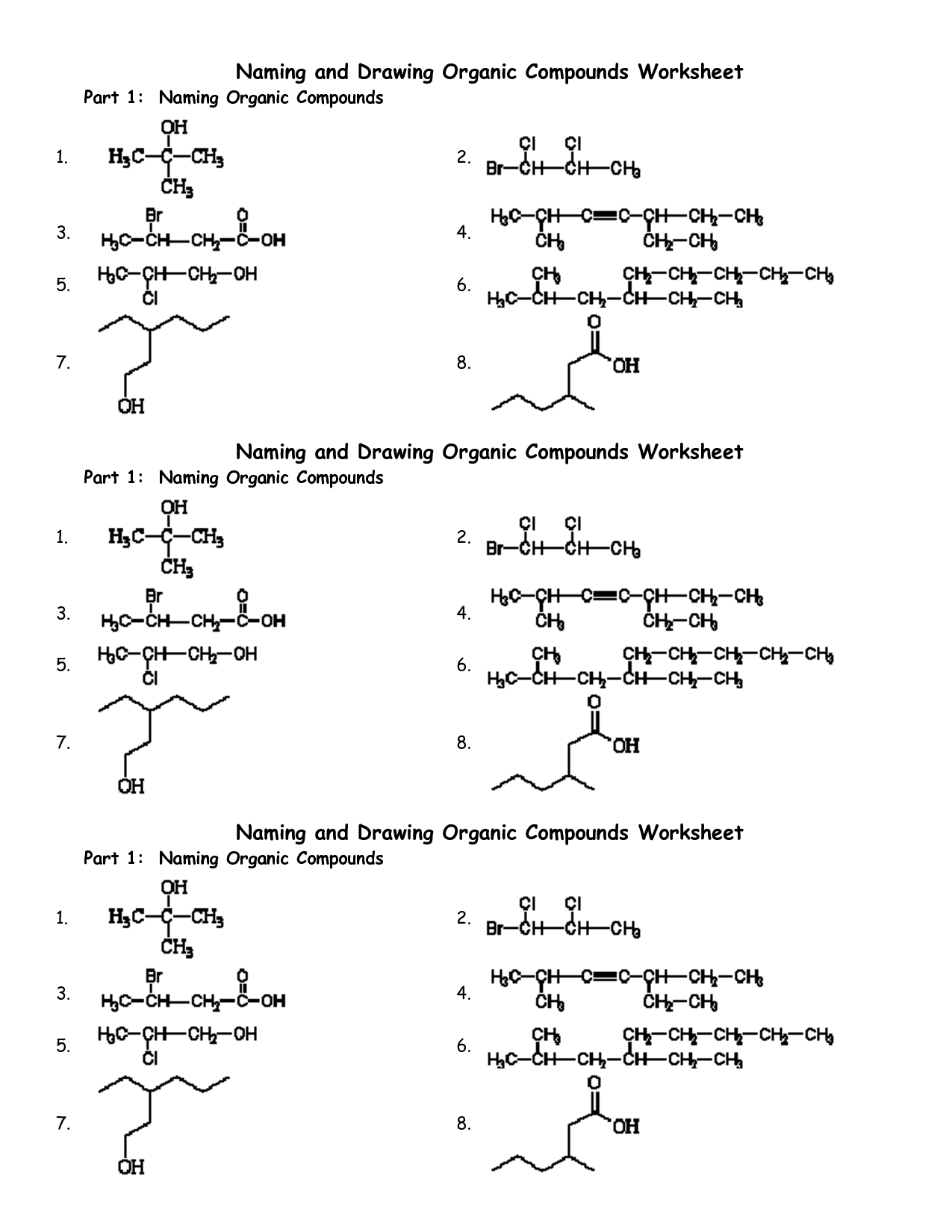
Naming Organic Compounds Worksheet Answer
Are you a chemistry student struggling to name organic compounds? Look no further than this Naming Organic Compounds Worksheet Answer blog post! We understand that identifying and correctly naming organic compounds can be a challenging task. This post is dedicated to providing you with a comprehensive answer key to a worksheet that will help you practice and improve your naming skills. So, if you're looking for a convenient way to test your knowledge and master the art of naming organic compounds, this blog post is for you.
Table of Images 👆
- Organic Compounds Worksheet
- Naming Organic Compounds Worksheet
- Naming Ionic Compounds Worksheet Answer Key
- Chemistry Naming Compounds Worksheet Answers
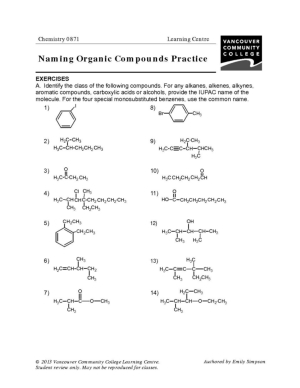
- Naming Organic Compounds Practice Worksheet
- Naming Organic Compounds Worksheet
- Naming Organic Compounds Practice Worksheet
- Organic vs Inorganic Compounds Worksheet
- Organic vs Inorganic Compounds Worksheet Answers
- Organic Chemistry Nomenclature Worksheets with Answers
- Molecular Compounds Worksheet
- Naming Ionic Compounds Worksheet Answer Key
- Chemical Nomenclature Worksheet Answers
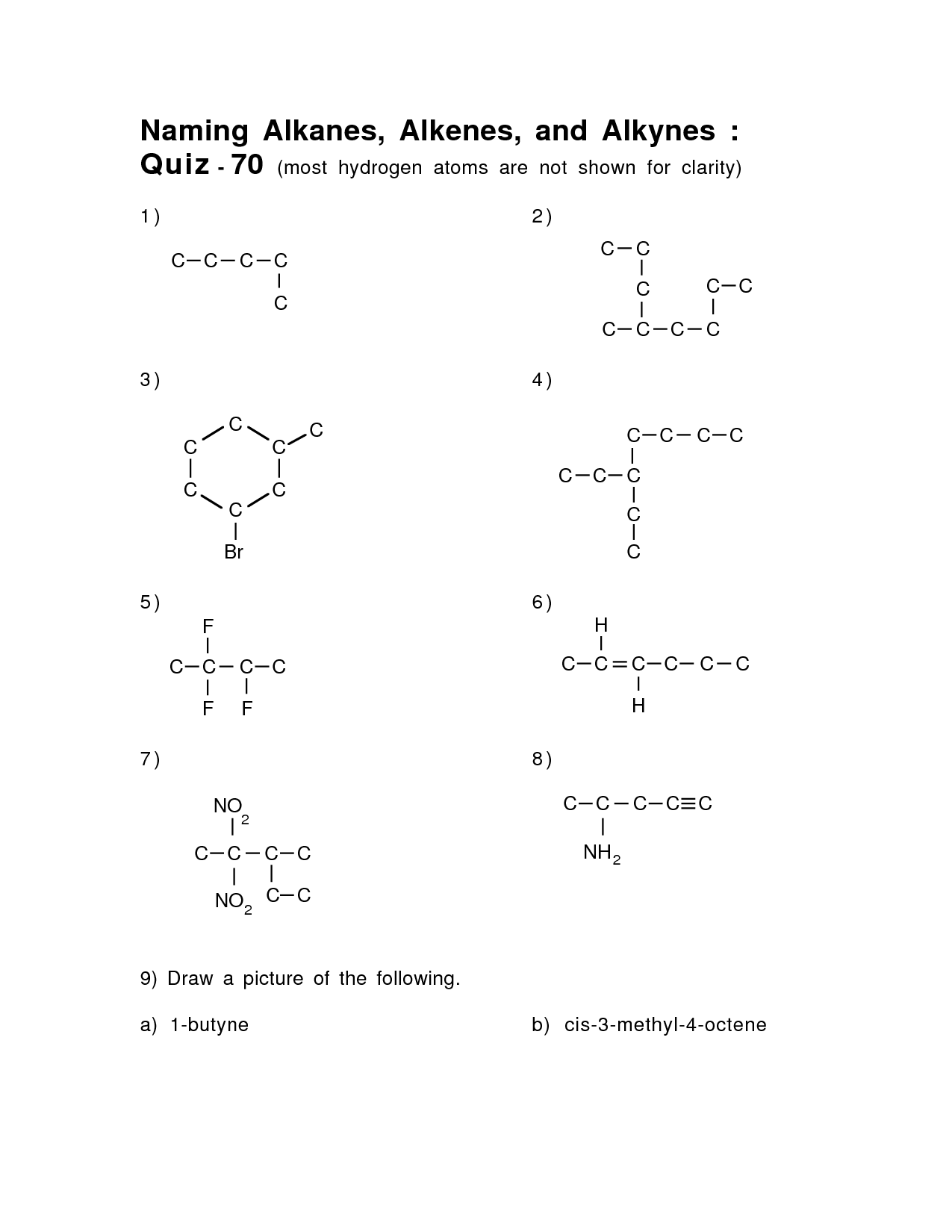
- Naming Alkanes Alkenes and Alkynes
- Naming Organic Compounds Practice Worksheet
- Organic Chemistry Nomenclature Worksheets with Answers
- Organic Chemistry Functional Groups Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the naming system used for organic compounds?
The naming system used for organic compounds is called the IUPAC (International Union of Pure and Applied Chemistry) nomenclature. This system provides a set of rules and guidelines for naming organic compounds based on the structure and functional groups present in the molecule. The IUPAC system aims to create a systematic and standardized way of naming organic compounds to ensure clarity and consistency in chemical communication.
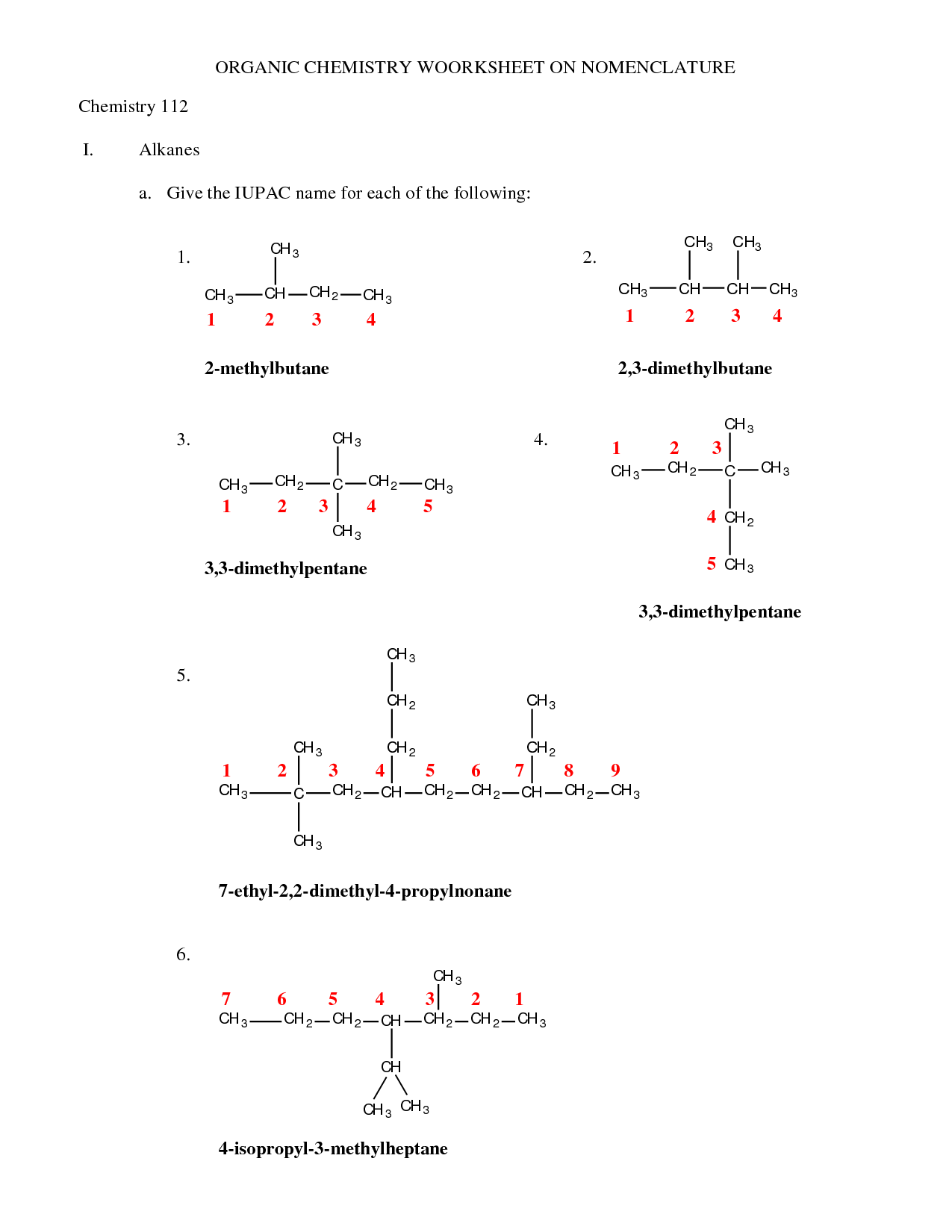
How are alkanes named?
Alkanes are named using the International Union of Pure and Applied Chemistry (IUPAC) system, which involves identifying the longest continuous carbon chain in the molecule and naming the alkane based on the number of carbon atoms in that chain. If there are branches or substituents attached to the carbon chain, their positions are indicated using locants (numbers) and their names are added as prefixes. The names of alkanes end with the suffix "-ane" to indicate it is an alkane.
What are substituents in organic compound names?
Substituents in organic compound names are functional groups or individual atoms that replace hydrogen atoms in a hydrocarbon molecule. These substituents can impact the chemical properties and reactivity of the compound. By naming these substituents using prefixes and suffixes based on the number of carbon atoms and the type of functional group, chemists can accurately describe the structure and composition of organic compounds.
How are alkenes named?
Alkenes are named based on the number of carbon atoms in the longest continuous chain containing the double bond. The parent name is derived from the number of carbons in the chain, with "-ene" added as the suffix to denote the presence of a double bond. The main chain is numbered to give the double bond the lowest possible carbon number. Any substituents or functional groups are then indicated by their location and name using prefixes such as "chloro" or "methyl.
What are functional groups in organic compounds?
Functional groups in organic compounds are specific groups of atoms that determine the chemical reactivity and properties of the molecule. They give organic molecules their unique characteristics and can participate in reactions by adding or removing particular functional groups. Common functional groups include hydroxyl (OH), carbonyl (C=O), amino (NH2), and carboxyl (COOH), among others. These functional groups play a crucial role in governing the behavior and interactions of organic compounds in various chemical processes.
How are alcohols named?
Alcohols are named by identifying the longest carbon chain attached to the hydroxyl group (OH) and then adding the suffix "-ol" to the root of the name of the parent alkane. The position of the hydroxyl group on the carbon chain is indicated by a number, with the number used to give the hydroxyl carbon the lowest possible number. Additionally, if there are other functional groups present, the alcohol group is given priority in naming.
How are ethers named?
Ethers are named by listing the two alkyl or aryl groups attached to the oxygen atom in alphabetical order followed by the word "ether." If the groups are the same, the prefix di-, tri-, etc., is used to indicate the number of groups, and the name of the alkyl or aryl group is only mentioned once. For example, CH3CH2OCH2CH3 is named ethyl methyl ether.
How are carboxylic acids named?
Carboxylic acids are named by replacing the "-e" ending of the corresponding alkane with "-oic acid." The parent chain for naming is the longest carbon chain that contains the carboxylic acid functional group, with the carboxyl carbon being carbon 1. If there are substituents present, they are numbered to give the carboxyl carbon the lowest possible number. Additionally, common names are often used for simple carboxylic acids, such as formic acid (methanoic acid) and acetic acid (ethanoic acid).
What is the difference between primary, secondary, and tertiary carbon atoms?
Primary carbon atoms are bonded to one other carbon atom, secondary carbon atoms are bonded to two other carbon atoms, and tertiary carbon atoms are bonded to three other carbon atoms. The main difference lies in the number of carbon atoms to which each type of carbon atom is directly bonded.
How are amides named?
Amides are named by taking the name of the corresponding carboxylic acid and replacing the -oic acid ending with -amide. For example, ethanoic acid becomes acetamide. If the amide is a derivative of a secondary amine, the prefix N- is added before the amide name. If it is a tertiary amide, the prefix N, N- is added.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments