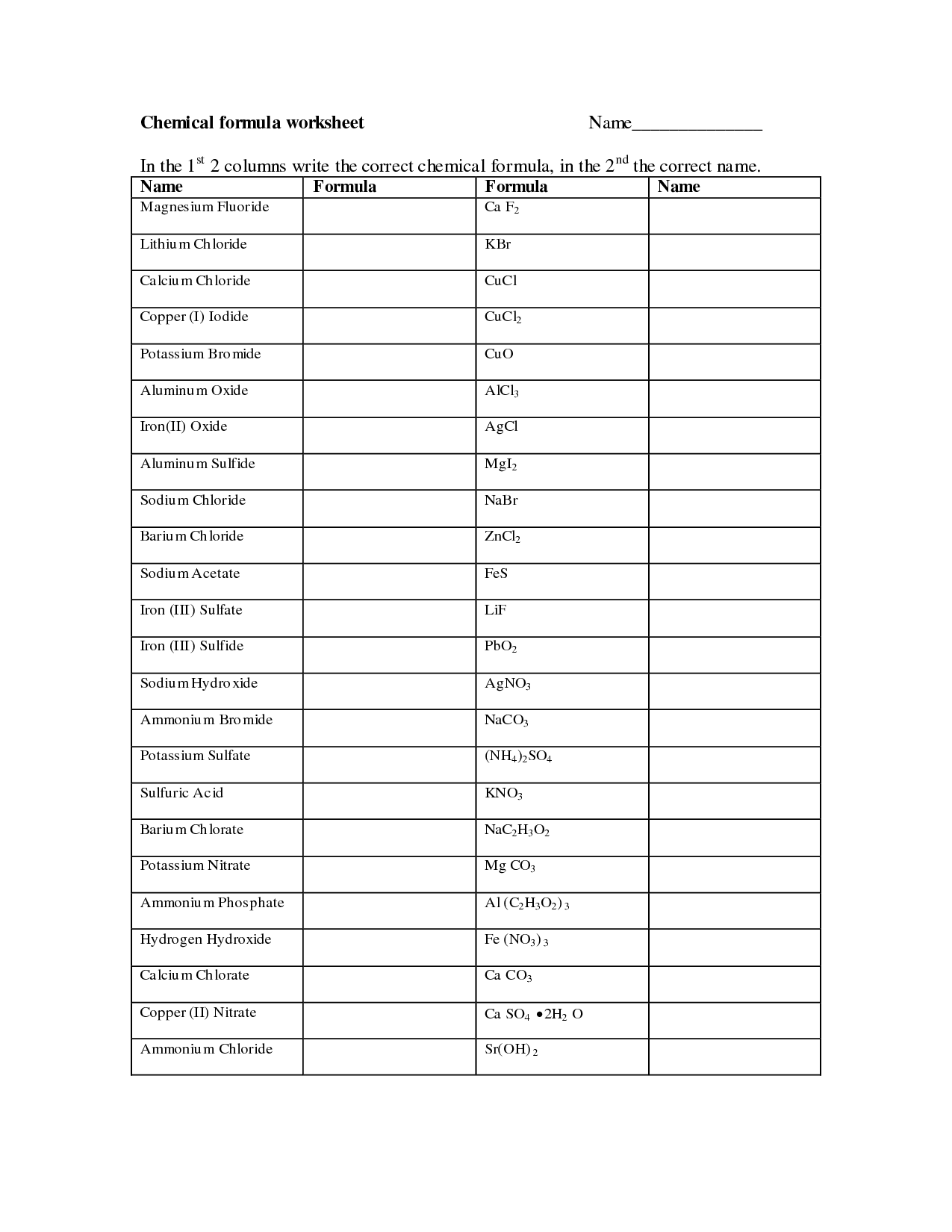
Naming Molecular Formula Worksheet
Molecular formulas are an essential part of understanding and working with chemical compounds. They provide key information about the types and numbers of atoms present in a molecule. If you are searching for a reliable and comprehensive resource to practice naming molecular formulas, you've come to the right place! Our Naming Molecular Formula Worksheet is perfect for students, chemistry enthusiasts, or anyone looking to enhance their knowledge in this subject.
Table of Images 👆
- Chemical Nomenclature Worksheet
- Molecular and Empirical Formula Worksheet Answers
- Naming Ionic Compounds Worksheet Answer Key
- Chemical Formula Writing Worksheet
- Naming Ionic Compounds Worksheet Answer Key
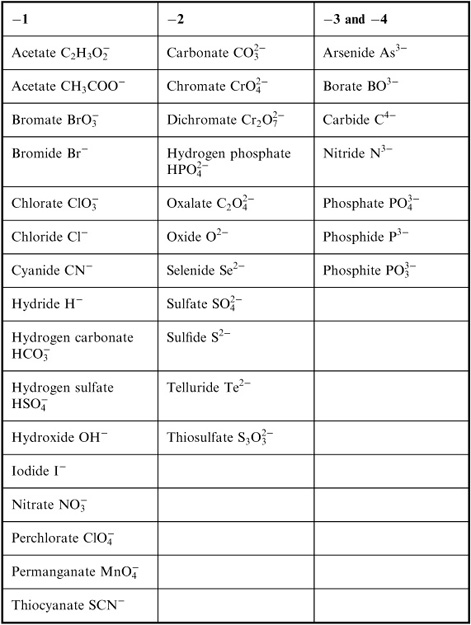
- Common Ionic Compounds List
- Binary Ionic Compounds Worksheet Answers
- Balancing Chemical Equations Worksheet Answer Key
- Naming Ionic Compounds Worksheet Answers
- Chemical Word Equations Worksheet Answers
- Organic Chemistry Functional Groups
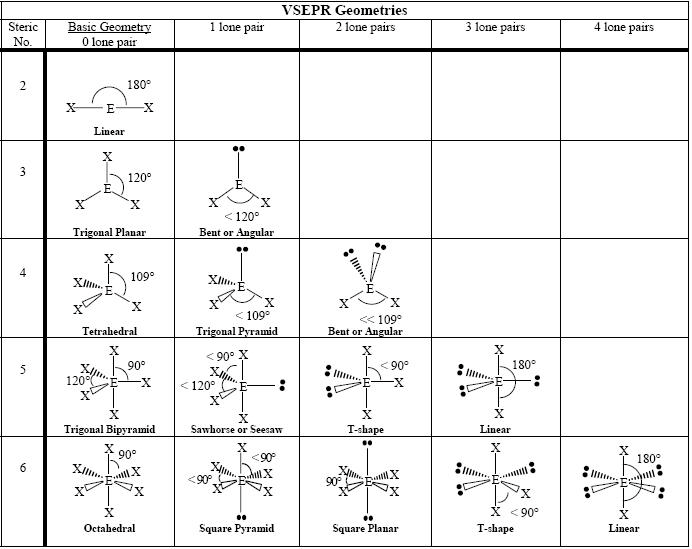
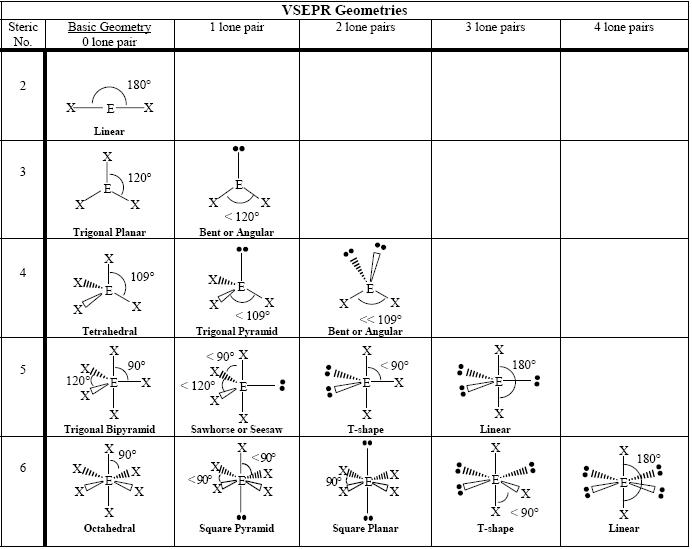
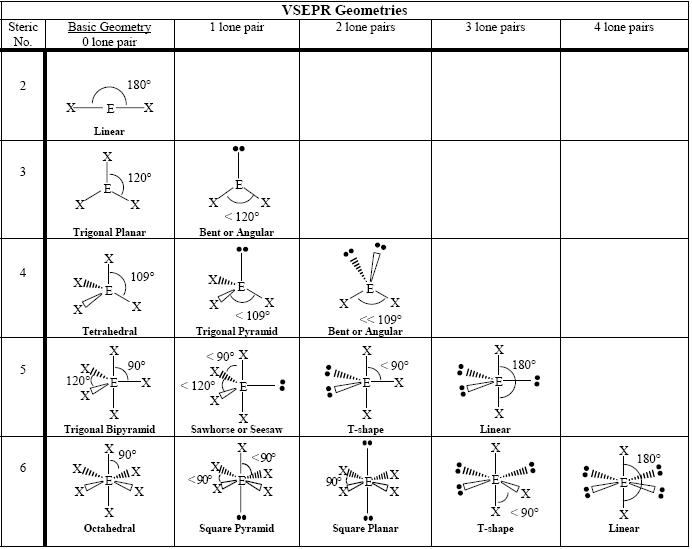
- VSEPR Molecular Geometry Chart
- VSEPR Molecular Geometry Chart
- VSEPR Molecular Geometry Chart
- VSEPR Molecular Geometry Chart
- VSEPR Molecular Geometry Chart
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is a molecular formula?
A molecular formula is a representation of the number and types of atoms present in a molecule. It shows the exact number of each type of atom in a molecule but does not provide information about how the atoms are arranged or bonded to each other within the molecule.
How do you determine the molecular formula of a compound?
To determine the molecular formula of a compound, you first need to know the empirical formula, which gives the simplest ratio of atoms present in the compound. Then, you can calculate the molar mass of the compound and compare it to the empirical formula to find the integer multiplier that gives the correct molecular formula. This multiplier represents how many times the empirical formula should be multiplied to achieve the correct molecular formula, revealing the specific arrangement of atoms in the compound.
What information does a molecular formula provide about a compound?
A molecular formula provides the specific number of each type of atom present in a compound. This information helps identify the elements composing the compound and their relative proportions, allowing for the determination of the compound's molecular structure and properties.
Why is it important to accurately name the molecular formula of a compound?
Accurately naming the molecular formula of a compound is essential because it provides a precise way to identify and communicate the composition and structure of the compound. This information is crucial for scientific research, education, and practical applications. It helps to ensure that everyone involved has a clear understanding of the compound being discussed, enabling effective communication and accurate interpretation of data and results. Additionally, correct naming ensures consistency and prevents confusion in the scientific community, which is crucial for advancing knowledge and discoveries.
How do you determine the number of each element present in a molecular formula?
To determine the number of each element present in a molecular formula, you simply need to count the number of atoms for each element listed in the formula. The subscripts next to each element indicate how many atoms of that element are present. For example, in the molecular formula H2O, there are 2 hydrogen atoms and 1 oxygen atom. By counting the number of each element's atoms, you can find the composition of the molecule.
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest ratio of the elements in a compound, while a molecular formula shows the exact number of each element present in a molecule. The empirical formula provides the relative proportions of atoms in a compound, while the molecular formula gives the actual number of each type of atom in a molecule.
How do you convert an empirical formula to a molecular formula?
To convert an empirical formula to a molecular formula, you need to know the molar mass of the compound. Divide the molecular mass of the compound by the molar mass of the empirical formula to determine the ratio between the empirical formula and the molecular formula. Finally, multiply the subscripts in the empirical formula by this ratio to obtain the molecular formula.
Can compounds have multiple molecular formulas?
No, compounds cannot have multiple molecular formulas. A compound always has a specific combination of elements in a fixed ratio, giving it a distinct molecular formula that represents the unique arrangement of atoms within the compound. Changing the molecular formula would result in a different compound with different properties and characteristics.
What role does stoichiometry play in determining the molecular formula?
Stoichiometry plays a crucial role in determining the molecular formula by providing the ratio of atoms in a compound. By analyzing the proportions of elements involved in a reaction or compound, stoichiometry helps identify the exact number of atoms and their types present in the molecular formula. This quantitative relationship enables chemists to determine the molecular formula by understanding how different elements combine and interact in a compound.
How can you confirm the accuracy of a molecular formula?
To confirm the accuracy of a molecular formula, one can utilize analytical techniques such as mass spectrometry, nuclear magnetic resonance spectroscopy, and elemental analysis. Mass spectrometry provides information on the exact mass of the compound, while NMR spectroscopy reveals the connectivity of atoms in the molecule. Elemental analysis helps determine the empirical formula by quantifying the elements present in the compound. By comparing the experimental data obtained from these techniques to the theoretical molecular formula, one can verify the accuracy of the molecular formula.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments