Naming Molecular Compounds Worksheet Answers
If you're searching for reliable answers to your naming molecular compounds worksheet, you've come to the right place. This blog post will provide you with detailed and accurate solutions to help you understand the nuances of naming chemical compounds.
Table of Images 👆
- Binary Ionic Compounds Worksheet 1 Answers
- Mixed Ionic Covalent Compound Naming
- Naming Covalent Compounds Worksheet Key
- Naming Acids Worksheet Answer Key
- Writing Ionic Compound Formula Worksheet Answers
- Binary Molecular Compounds Worksheet Answers
- Polyatomic Ions Answer Key POGIL
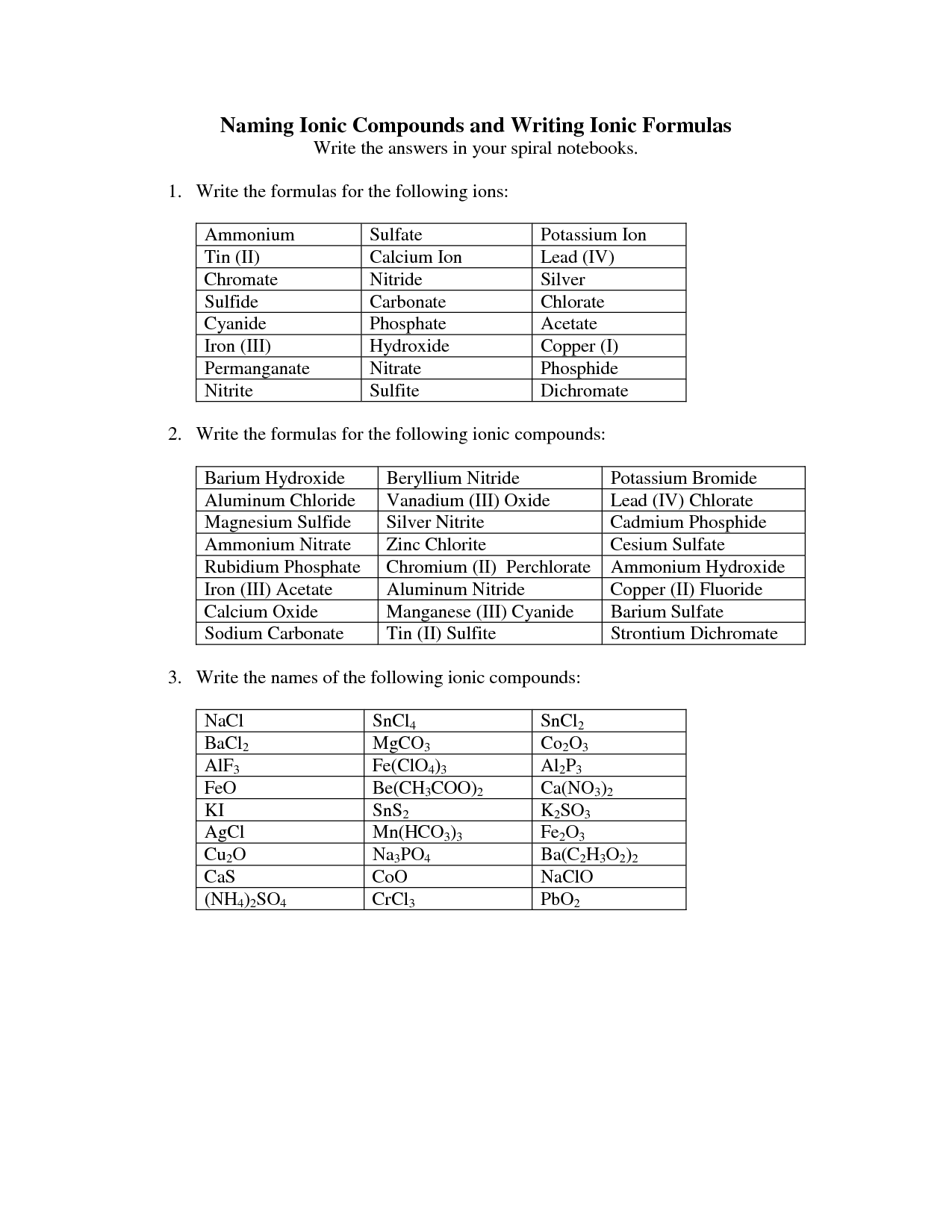
- Naming Ionic Compounds Worksheet Answer Key
- Common Polyatomic Ions Worksheet Answers
- Naming Ionic Compounds Worksheet
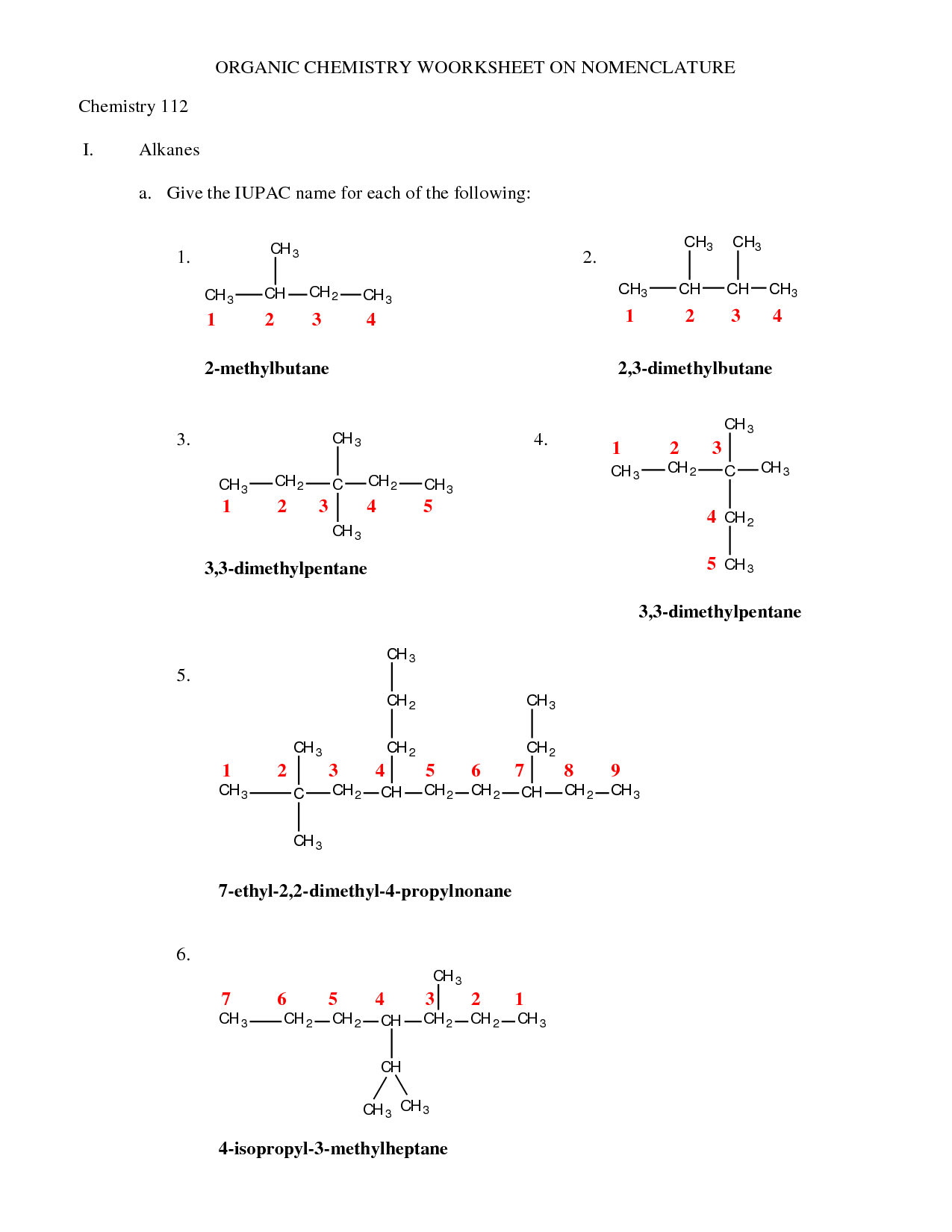
- Naming Organic Compounds Practice Worksheet
- Nomenclature Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
Why do we use prefixes in naming molecular compounds?
We use prefixes in naming molecular compounds to indicate the number of atoms of each element present in the compound. This helps to provide specific and clear information about the molecular composition and structure of the compound, allowing for accurate communication and understanding in the field of chemistry.
What is the prefix used when there is only one atom of the first element?
The prefix "mono-" is used when there is only one atom of the first element in a chemical compound.
How do you determine the prefix for the second element in a molecular compound?
To determine the prefix for the second element in a molecular compound, you need to use the prefixes based on the number of atoms of each element present in the compound. The prefixes used for naming molecular compounds are: mono- for one, di- for two, tri- for three, tetra- for four, penta- for five, hexa- for six, hepta- for seven, octa- for eight, nona- for nine, and deca- for ten. Use these prefixes to indicate the number of atoms of the second element in the compound, followed by the element's name with its ending changed to "-ide.
What is the name of N2O3?
The name of N2O3 is dinitrogen trioxide.
What is the name of CO2?
The name of CO2 is carbon dioxide.
How do you name a molecular compound when there is a metal involved?
When naming a molecular compound that includes a metal, the metal is typically named first, followed by the nonmetal with an -ide ending. Make sure to specify the charge of the metal using Roman numerals in parentheses after the metal's name. This naming system helps to indicate the specific composition and charge of the compound.
What is the name of PCl5?
The name of PCl5 is phosphorus pentachloride.
How do you name a molecular compound when there is a polyatomic ion involved?
When naming a molecular compound that includes a polyatomic ion, simply treat the polyatomic ion as a single unit and name the compound following the standard rules for naming molecular compounds. The name of the polyatomic ion should not change. Just name the other element or elements in the compound and use prefixes to indicate the number of atoms as needed, if they are present.
What is the name of H2O?
The name of H2O is water.
How do you name a binary acid as a molecular compound?
To name a binary acid as a molecular compound, you use the prefix "hydro-" followed by the root of the nonmetal element in the compound with the ending "-ic acid." For example, HCl would be named hydrochloric acid.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments