Naming Binary Compounds Worksheet Answers
This blog post provides the answers for the "Naming Binary Compounds Worksheet," catering to chemistry students and anyone seeking to enhance their understanding of chemical nomenclature. The worksheet features various binary compounds, and the answers will help reinforce knowledge of naming conventions and improve proficiency in this subject area.
Table of Images 👆
- Binary Ionic Compounds Worksheet 1 Answers
- Naming Acids Worksheet Answer Key
- Writing Ionic Compound Formula Worksheet Answers
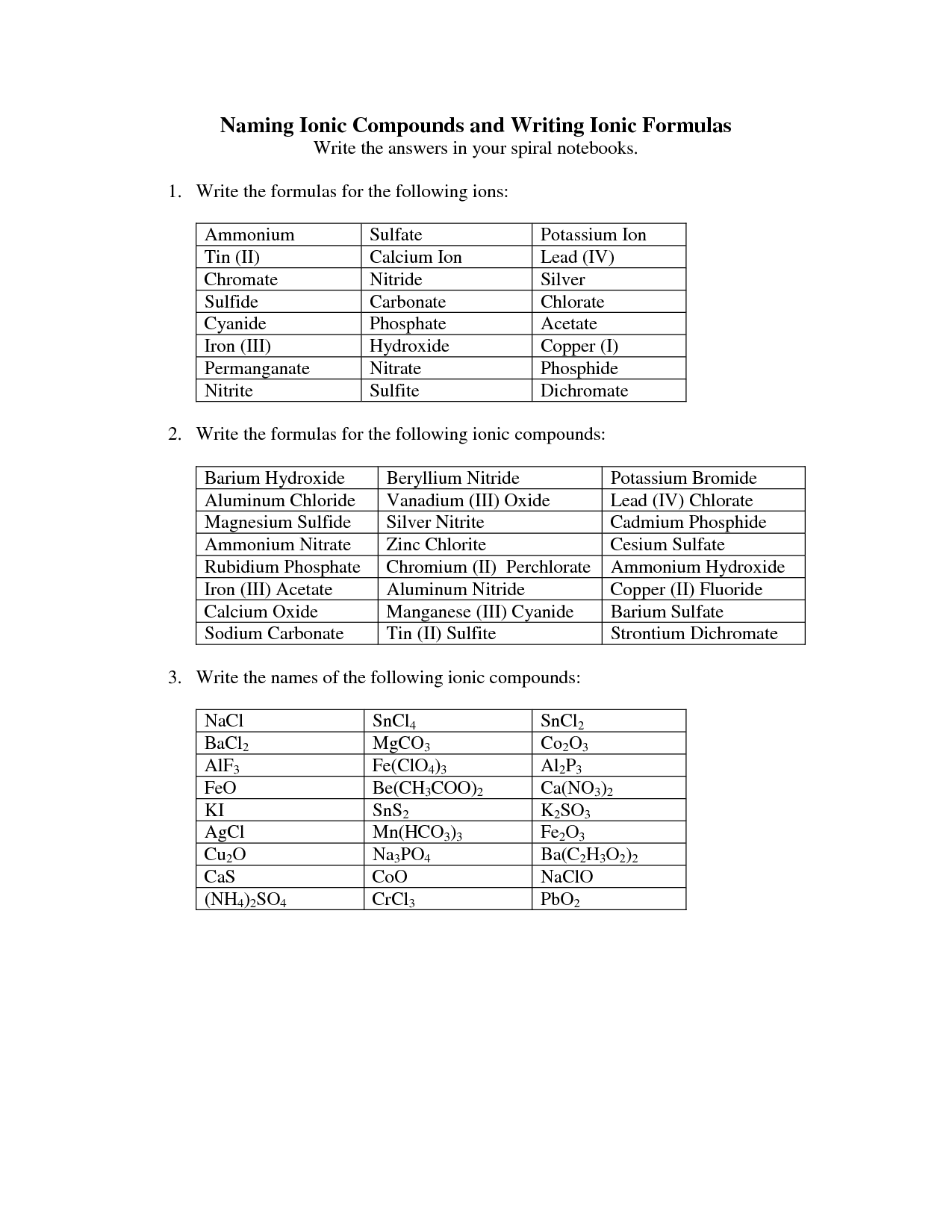
- Naming Ionic Compounds Worksheet Answers
- Naming Ionic Compounds Worksheet
- Formulas for Covalent Compounds for Antimony Tribromide
- Common Polyatomic Ions Worksheet Answers
- Binary Ionic Compounds Worksheet Answers
- Compounds Polyatomic Ions Ionic
- Binary Molecular Compounds Worksheet Answers
- Naming Organic Compounds Practice Worksheet
- Chemistry Writing Chemical Formulas
- Chemistry Writing Chemical Formulas
- Chemistry Writing Chemical Formulas
- Chemistry Writing Chemical Formulas
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the naming convention for binary compounds?
Binary compounds are named using the names of the two elements they are made of, with the name of the metal coming first followed by the nonmetal with its suffix changed to "-ide." For example, NaCl is named sodium chloride, where sodium is the metal and chlorine is the nonmetal.
How do you name binary compounds composed of two nonmetals?
To name binary compounds composed of two nonmetals, you use the naming system for covalent compounds. Start by naming the first element in the formula and then the second element with an -ide suffix. Remember to use prefixes to indicate the number of atoms of each element present if necessary. For example, CO is named carbon monoxide, and N2O5 is named dinitrogen pentoxide.
How do you name binary compounds composed of a metal and a nonmetal?
Binary compounds composed of a metal and a nonmetal are named by first writing the name of the metal followed by the name of the nonmetal with the ending changed to "-ide." For example, sodium chloride is formed from sodium (metal) and chlorine (nonmetal). The name of the metal is written first, followed by the nonmetal with the ending changed to "-ide," hence "sodium chloride.
What is the naming convention for binary compounds with a metal that forms multiple ions?
For binary compounds with a metal that forms multiple ions, the naming convention involves using Roman numerals in parentheses to indicate the charge of the metal ion. The Roman numeral corresponds to the charge of the metal ion in the compound. For example, iron can form Fe^2+ or Fe^3+ ions, so iron(II) oxide would be FeO for the Fe^2+ ion, and iron(III) oxide would be Fe2O3 for the Fe^3+ ion.
How do you name binary compounds with a metal that forms multiple ions?
To name binary compounds with a metal that forms multiple ions, you use Roman numerals in parenthesis to indicate the charge of the metal ion. The Roman numeral corresponds to the charge of the metal ion in the compound. For example, if you have FeCl2, the compound is named iron(II) chloride because iron can form two different ions with different charges.
What are the rules for naming binary compounds with transition metals?
When naming binary compounds with transition metals, the transition metal is named first followed by the non-metal element with its stem and an "-ide" ending. The charge of the transition metal must be indicated using Roman numerals in parentheses after the metal name. For example, iron(II) chloride is formed by the transition metal iron with a +2 charge bonding with chloride. If the transition metal can have multiple charges, then the charge must be specified using the Roman numeral system to avoid confusion.
How do you name binary compounds with polyatomic ions?
To name binary compounds with polyatomic ions, simply follow the same naming convention as for binary compounds with single ions. Start by identifying the cation (positively charged ion) and the anion (negatively charged ion), then write the name of the cation followed by the name of the polyatomic anion. Additionally, you may need to refer to a list of common polyatomic ions to determine the correct name. Remember to balance the charges between the cation and anion to determine the appropriate subscripts, if necessary.
How do you name binary compounds with polyatomic ions and a metal that forms multiple ions?
To name binary compounds with polyatomic ions and a metal that forms multiple ions, simply name the metal cation first, followed by the name of the polyatomic ion. If the metal forms multiple ions, you will need to include the charge of the metal cation as a roman numeral in parentheses after the metal name. For example, iron(III) sulfate is a binary compound of the iron(III) cation and the sulfate polyatomic ion.
How do you name binary compounds containing a polyatomic ion in the formula?
To name binary compounds containing a polyatomic ion in the formula, you first identify the positive element (usually a metal) and the negative polyatomic ion. Then you only need to name the metal followed by the name of the polyatomic ion as it is, without changing its name. For example, NaNO3 is named sodium nitrate where "sodium" is the metal and "nitrate" is the polyatomic ion.
What are some common mistakes to avoid when naming binary compounds?
Some common mistakes to avoid when naming binary compounds are mixing up the prefixes for the elements (such as using "mono" instead of "di" for compounds with two atoms of the first element), failing to reduce the subscripts in the chemical formula to their simplest ratio for naming purposes, and forgetting to change the ending of the second element to "ide." It's also important to remember that when the first element is a metal, Roman numerals may be needed to indicate the charge of the metal ion in the compound.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments