Mole Ratio Worksheet Answers
Are you a chemistry student looking for Mole Ratio Worksheet answers? Look no further! In this post, we will provide you with a detailed explanation of mole ratio and how it is used in chemistry, along with answers to a variety of worksheet questions. Whether you are studying for an exam or simply trying to reinforce your understanding of mole ratios, this blog post is for you.
Table of Images 👆
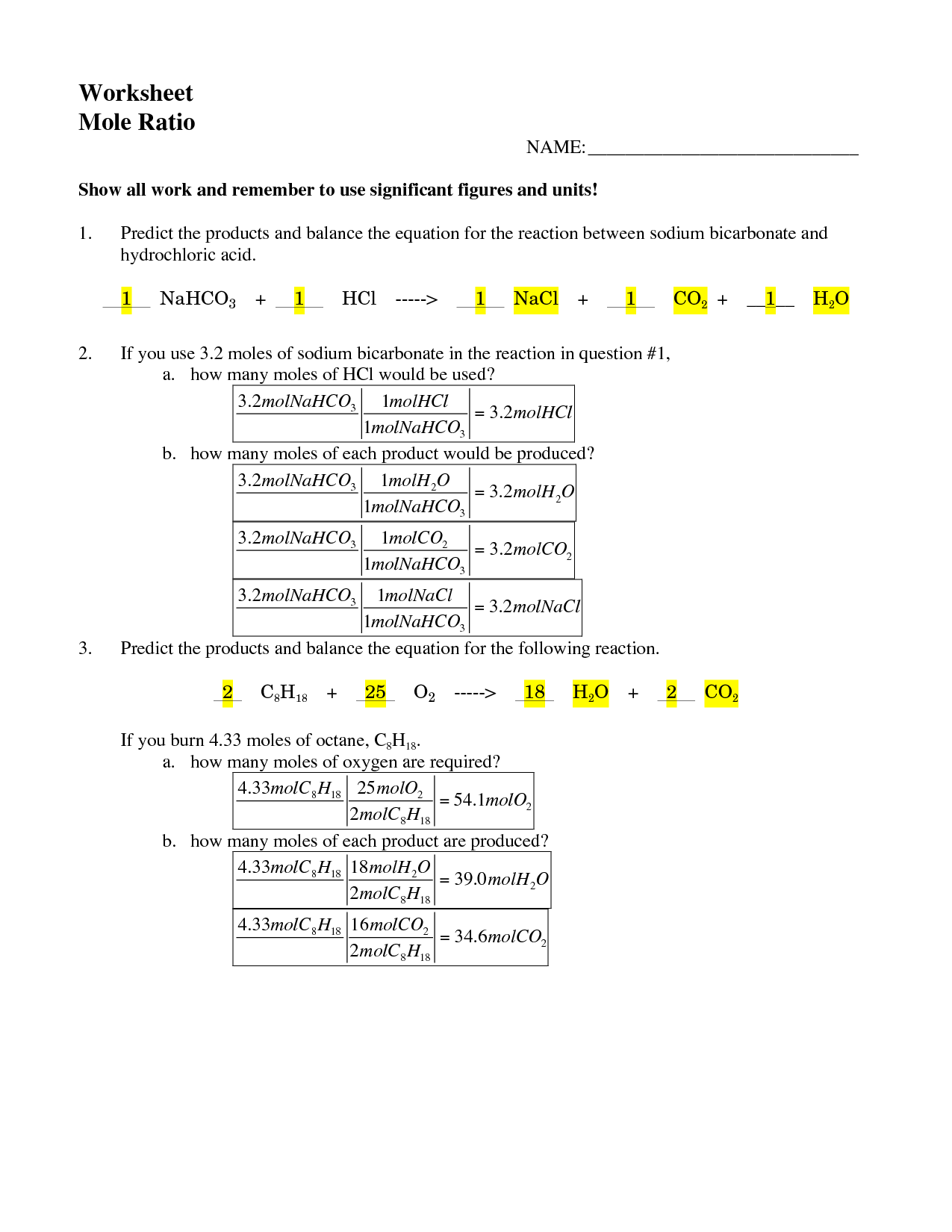
- Mole Ratio Worksheet Answer Key
- Mole Ratio Worksheet Answer Key
- Mole Ratio Worksheet Answer Key
- Mole Ratio Worksheet Answer Key
- Mole Ratio Worksheet Answer Key
- Mole Ratio Worksheet Answer Key
- Mass to Mole Stoichiometry Worksheet Answer Key
- Moles and Mass Worksheet Answers
- Mole Ratios POGIL Answer Key
- Mole Ratios POGIL Answer Key
- Chapter 8 Covalent Bonding Worksheet Answer Key
- Scientific Report Template
- Chemistry Mole Ratios POGIL Answer Key
- Mole Conversion Worksheet with Answers
- Chemistry Mole Concept Worksheet Answers
- Mole Ratio Worksheet Answer Key
- Energy Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is a mole ratio?
A mole ratio refers to the ratio between the number of moles of two substances in a chemical reaction. It is obtained by comparing the coefficients of the substances in a balanced chemical equation. Mole ratios are crucial in stoichiometry for determining the quantities of reactants and products involved in a reaction.
How is a mole ratio determined?
A mole ratio is determined by comparing the coefficients of the reactants and products in a balanced chemical equation. These coefficients represent the number of moles of each substance involved in a reaction. By comparing the ratios of these coefficients, you can determine the mole ratio between different substances in the chemical reaction.
How can mole ratios be used in chemical equations?
Mole ratios in chemical equations are used to determine the relationships between different reactants and products involved in a chemical reaction. By comparing the coefficients of the reactants and products in a balanced chemical equation, mole ratios can be established to calculate the amount of one substance that is consumed or produced in relation to another substance. This information is crucial for determining the optimal amounts of reactants required for a reaction, predicting the yield of a product, and understanding the stoichiometry of the reaction.
What are the units of mole ratio?
Mole ratio does not have any units because it is a ratio of the amount of moles of each substance in a chemical reaction. Mole ratio is a dimensionless quantity that is used to express the proportion between reactants and products in a balanced chemical equation.
How does a mole ratio relate to stoichiometry?
A mole ratio in stoichiometry is the ratio of moles of one substance to moles of another substance in a balanced chemical equation. This ratio is crucial for determining the quantities of reactants and products in a chemical reaction. By using the mole ratio, one can convert between moles of different substances involved in the reaction, allowing for precise calculations of reactants needed or products formed. Ultimately, the mole ratio is a fundamental concept in stoichiometry as it enables the conversion of quantities of substances in chemical reactions.
Can mole ratios be used to convert between different substances in a reaction?
Yes, mole ratios can be used to convert between different substances in a reaction. By comparing the coefficients of the substances in a balanced chemical equation, one can determine the ratio of moles of one substance to another involved in the reaction. This ratio allows for the conversion of moles of one substance to moles of another, which is essential for performing stoichiometry calculations in chemistry.
How is a mole ratio calculated from a balanced chemical equation?
To calculate a mole ratio from a balanced chemical equation, you must look at the coefficients of the reactants and products in the equation. The mole ratio is the ratio of moles of one substance to another in a chemical reaction, based on the coefficients. For example, in the equation 2H₂ + O₂ → 2H₂O, the mole ratio of hydrogen to water is 2:2 or 1:1 because the coefficients of both substances are the same. The coefficients represent the number of moles involved in the reaction, allowing you to determine the mole ratio between different substances in the reaction.
How can mole ratios be used to calculate the quantity of a substance in a reaction?
Mole ratios can be used to calculate the quantity of a substance in a reaction by comparing the stoichiometric coefficients of the reactants and products in a balanced chemical equation. By converting the given quantity of one substance to moles and using the mole ratio between the reactant and the desired substance, the amount of the desired substance can be calculated. This method allows for precise control and prediction of the amounts of substances involved in a reaction based on the stoichiometry of the reaction.
Can mole ratios be used to determine the limiting reactant in a reaction?
Yes, mole ratios can be used to determine the limiting reactant in a reaction by comparing the actual number of moles of each reactant present to the stoichiometrically balanced coefficients in the chemical equation. Whichever reactant produces the least amount of product based on the mole ratios is the limiting reactant.
How are mole ratios helpful in understanding the relationships between reactants and products in a chemical reaction?
Mole ratios are helpful in understanding the relationships between reactants and products in a chemical reaction because they provide a way to quantify the amounts of substances involved in the reaction. By comparing the mole ratios of reactants and products, chemists can determine the stoichiometry of the reaction, predict how much product will be formed from a given amount of reactant, and identify the limiting reactant. This information is crucial for accurately balancing chemical equations and optimizing reaction conditions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


































Comments