Mass and Moles Worksheet Answer Key
Are you in need of a reliable answer key for your mass and moles worksheet? Look no further! We understand the importance of having accurate and comprehensive answers for these types of assignments. Whether you're a high school student studying chemistry, a college student taking an introductory science course, or even a teacher looking for a resource to guide your lesson plans, this mass and moles worksheet answer key is just what you need.
Table of Images 👆
- Mole Conversion Worksheet Answers
- Moles Molecules and Grams Worksheet
- Solution Stoichiometry Worksheet
- Mole to Mass Stoichiometry Problems Answers
- Mole and the Relative Mass POGIL Answers
- Worksheets Calculating Percent Composition Chemistry
- Series Parallel Circuits Worksheets and Answer Key
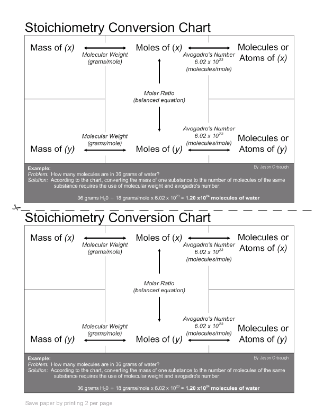
- Stoichiometry Conversion Chart
- Dimensional Analysis Worksheets with Answer Keys
- Lewis Dot Structure Worksheet Answers
- As Periodic Table of Elements
- As Periodic Table of Elements
- As Periodic Table of Elements
- As Periodic Table of Elements
- As Periodic Table of Elements
- As Periodic Table of Elements
- As Periodic Table of Elements
- As Periodic Table of Elements
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is mass?
Mass is a measure of the amount of matter in an object. It is a fundamental property of an object that determines the object's inertia and its gravitational attraction to other objects. The SI unit of mass is the kilogram (kg).
How is mass measured?
Mass is typically measured using a balance or a scale, where an object's weight is compared to a known standard, such as a set of calibrated weights or a reference object of known mass. The mass is then determined based on this comparison, with the unit of measurement often being grams or kilograms in the metric system.
What is the SI unit for mass?
The SI unit for mass is the kilogram (kg).
How is mass different from weight?
Mass is a measure of the amount of matter in an object, typically measured in units like kilograms or grams. Weight, on the other hand, is the force of gravity acting on an object's mass and is influenced by the acceleration due to gravity. So, while mass remains constant regardless of location, weight can vary depending on the gravitational pull at a specific location.
What is a mole?
A mole is a unit of measurement used in chemistry to represent the amount of a substance. One mole is equal to Avogadro's number, which is approximately 6.022 x 10^23 particles (atoms, molecules, ions, etc.) of that substance. It is a convenient way to count large numbers of particles in a manageable unit for calculations and comparisons in chemical reactions and equations.
How is a mole defined in relation to the Avogadro constant?
A mole is defined as the amount of substance that contains the same number of entities as there are atoms in 12 grams of carbon-12. This number is known as Avogadro's constant, which is approximately 6.022 × 10^23 entities per mole.
What is the molar mass?
The molar mass is the mass of one mole of a substance, expressed in grams per mole. It is calculated by summing the atomic masses of the atoms in a molecule and is a conversion factor that relates the mass of a substance to the number of moles present.
How is molar mass calculated?
Molar mass is calculated by summing the atomic masses of all the elements in a chemical compound, as given in the periodic table. The atomic masses are usually expressed in atomic mass units (amu) and can be found by multiplying the atomic mass of each element by the number of atoms of that element in the compound. The resulting total molar mass is expressed in grams per mole (g/mol).
What is the relationship between mass and moles in a chemical equation?
The relationship between mass and moles in a chemical equation is determined by the molar mass of a substance. Molar mass is the mass in grams of one mole of a substance. By knowing the molar mass of a substance, one can convert between mass and moles using the formula: moles = mass (g) / molar mass (g/mol). This relationship is crucial for balancing chemical equations and calculating stoichiometry in chemical reactions.
How can mass be converted into moles?
To convert mass into moles, you need to use the molar mass of the substance. First, determine the molar mass by adding up the atomic masses of the elements in the substance. Then, divide the given mass by the molar mass to find the number of moles using the formula: moles = mass (g) / molar mass (g/mol). This conversion allows you to relate the mass of a substance to its quantity in terms of moles.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments