Ionic and Covalent Compounds Worksheet
Worksheets are invaluable resources for both educators and students seeking a better understanding of ionic and covalent compounds. Designed specifically for middle school and high school science classes, these worksheets offer targeted practice and reinforcement of key concepts related to chemical bonding. With clear instructions and a variety of engaging exercises, students can enhance their knowledge and mastery of this fundamental topic.
Table of Images 👆
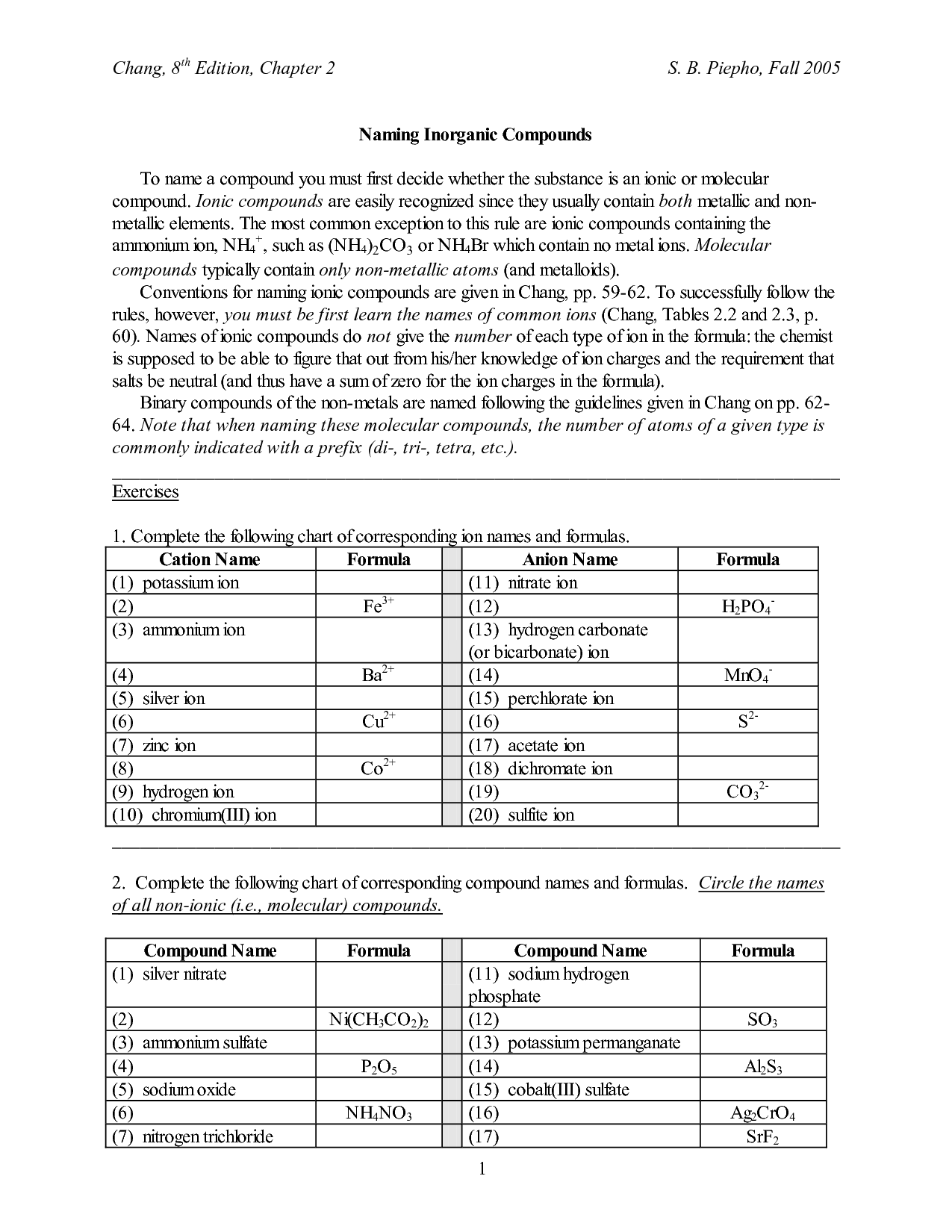
- Naming Ionic Compounds Worksheet Answer Key
- Naming Ionic Compounds Worksheet One
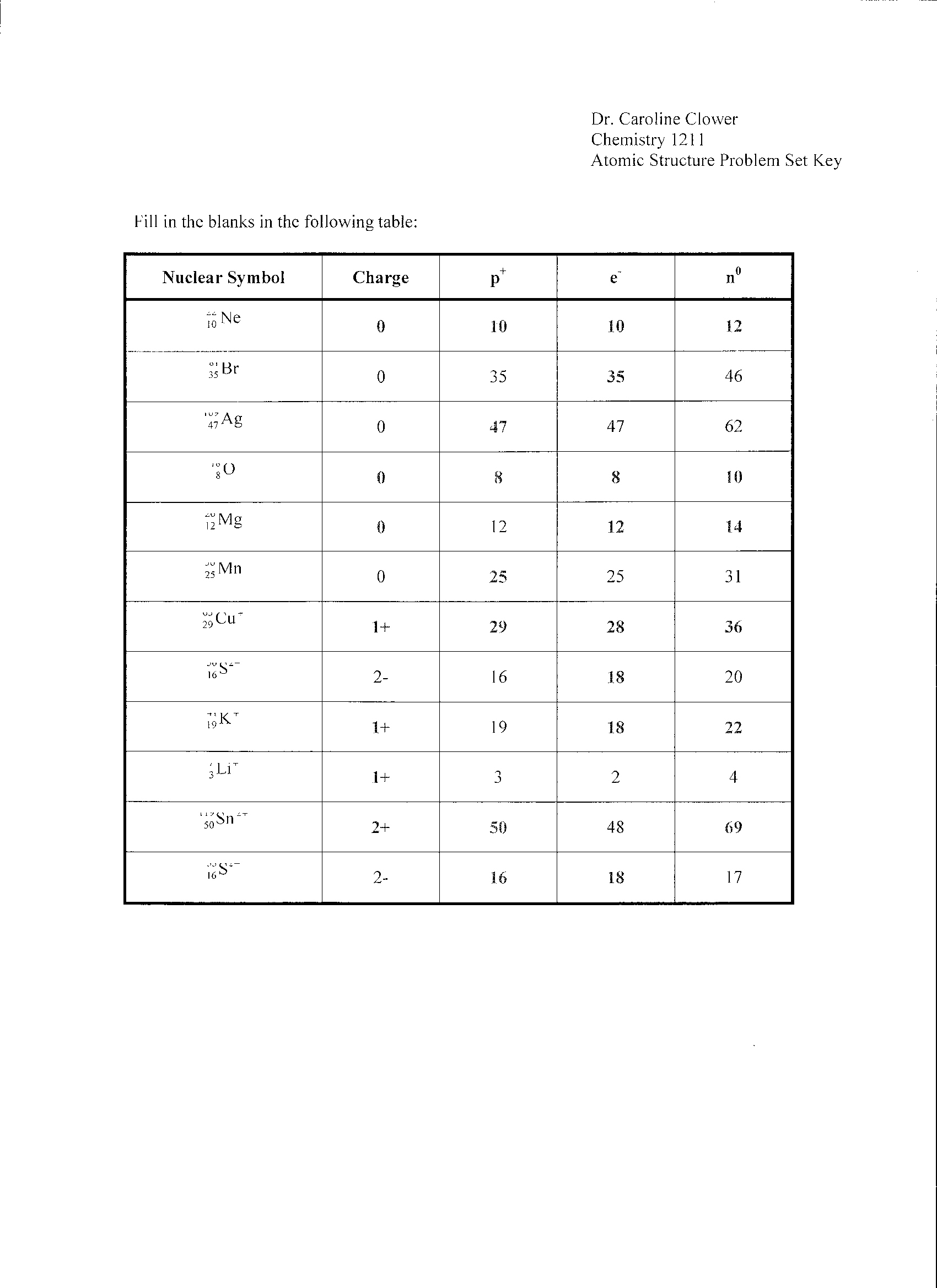
- Atomic Structure Worksheet Answers
- Naming Ionic Compounds Worksheet
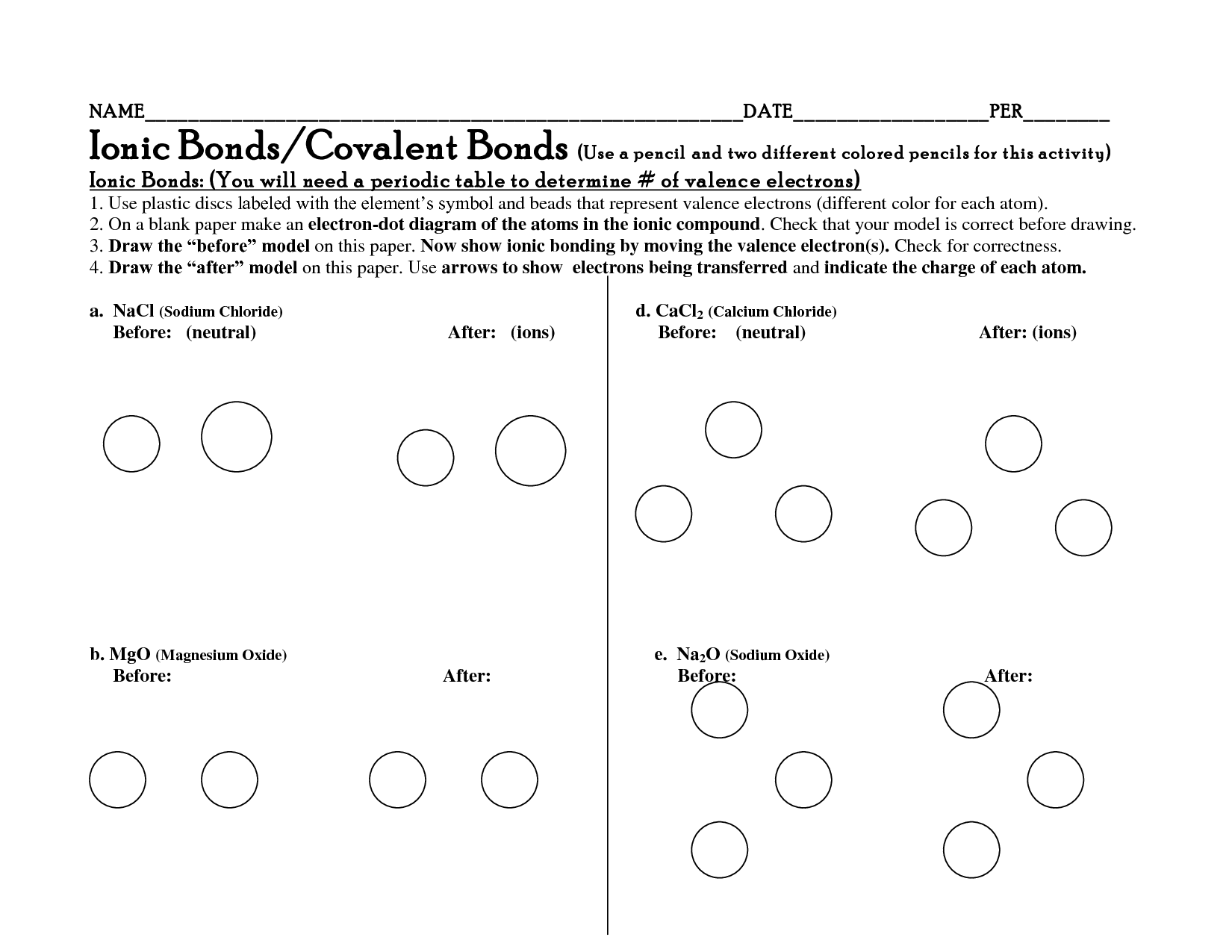
- Ionic and Covalent Bonds Activities
- Ionic and Covalent Bonds Worksheet
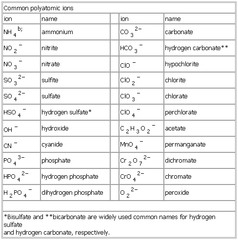
- Ionic Compounds Formulas and Names
- States and Capitals Test Printable
- Naming Binary Ionic Compounds Worksheet
- Binary Ionic Compounds Worksheet Answers
- Bond Length and Bond Energy Table
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is an ionic compound?
An ionic compound is a chemical compound composed of positively charged ions (cations) and negatively charged ions (anions) held together by electrostatic forces of attraction. These compounds typically form when a metal reacts with a non-metal, transferring electrons to one another to achieve a stable electron configuration. Ionic compounds usually have high melting and boiling points due to their strong ionic bonds.
What is a covalent compound?
A covalent compound is a chemical compound formed by the sharing of electron pairs between atoms, resulting in the formation of a stable molecule. These compounds typically involve nonmetal elements bonding together, such as in molecules like water (H2O) or methane (CH4), where the atoms are held together by strong covalent bonds.
How are ionic compounds formed?
Ionic compounds are formed through the transfer of electrons between a metal atom (which loses electrons to become a positive ion) and a nonmetal atom (which gains electrons to become a negative ion). These opposite charged ions are then attracted to each other by electrostatic forces, forming a stable ionic bond. This process allows the atoms to achieve a full outer shell of electrons, leading to a more stable configuration.
How are covalent compounds formed?
Covalent compounds are formed when two or more nonmetal atoms share electrons to achieve a stable electron configuration. This sharing of electrons creates a bond between the atoms, with each atom contributing one or more electrons to the shared pair. The atoms are held together by the strong electrostatic forces between the positively charged atomic nuclei and the negatively charged shared electrons, resulting in the formation of a covalent compound.
What type of bonding occurs in ionic compounds?
Ionic compounds form ionic bonds, which are characterized by the transfer of electrons from one atom to another. This transfer leads to the formation of positively charged ions (cations) and negatively charged ions (anions) that are held together by electrostatic attraction.
What type of bonding occurs in covalent compounds?
Covalent compounds exhibit covalent bonding, which involves the sharing of electrons between atoms to form a stable molecule. These bonds are formed when atoms with similar electronegativities share electrons in such a way that each atom achieves a full outer shell of electrons, resulting in a strong bond that holds the atoms together in the compound.
What are some properties of ionic compounds?
Ionic compounds are composed of positively and negatively charged ions held together by electrostatic forces. They typically have high melting and boiling points due to their strong ionic bonds. They are usually soluble in water and conduct electricity when dissolved or melted. Ionic compounds also tend to have a crystalline structure and can exhibit characteristics such as being brittle and easily cleaved.
What are some properties of covalent compounds?
Covalent compounds are typically formed between nonmetals and are characterized by the sharing of electrons between the atoms. These compounds have lower melting and boiling points compared to ionic compounds, are usually softer, and are poor conductors of electricity in their pure form. Covalent compounds often exist as discrete molecules and can exhibit a wide range of physical properties depending on the elements involved and the way the atoms are bonded together.
Give an example of an ionic compound.
Table salt, or sodium chloride (NaCl), is an example of an ionic compound. It is formed when a positively charged sodium ion (Na+) bonds with a negatively charged chloride ion (Cl-) through an ionic bond.
Give an example of a covalent compound.
Water (H2O) is a common example of a covalent compound. Two hydrogen atoms bond with one oxygen atom through covalent bonds, where they share electrons to form a stable molecule.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments