Ion Worksheet High School
Worksheets are an integral part of the learning process for high school students at Ion School. These handouts are designed to provide students with a structured way to practice and reinforce their understanding of various subjects and topics. From chemistry equations to mathematical formulas, worksheets serve as a valuable tool that enhances the learning experience for students at Ion School.
Table of Images 👆
- Nomenclature Worksheet 2 Answer Key
- Writing Ionic Compound Formula Worksheet Answers
- Chemistry Formula Writing Worksheet
- Solubility Rules Worksheet
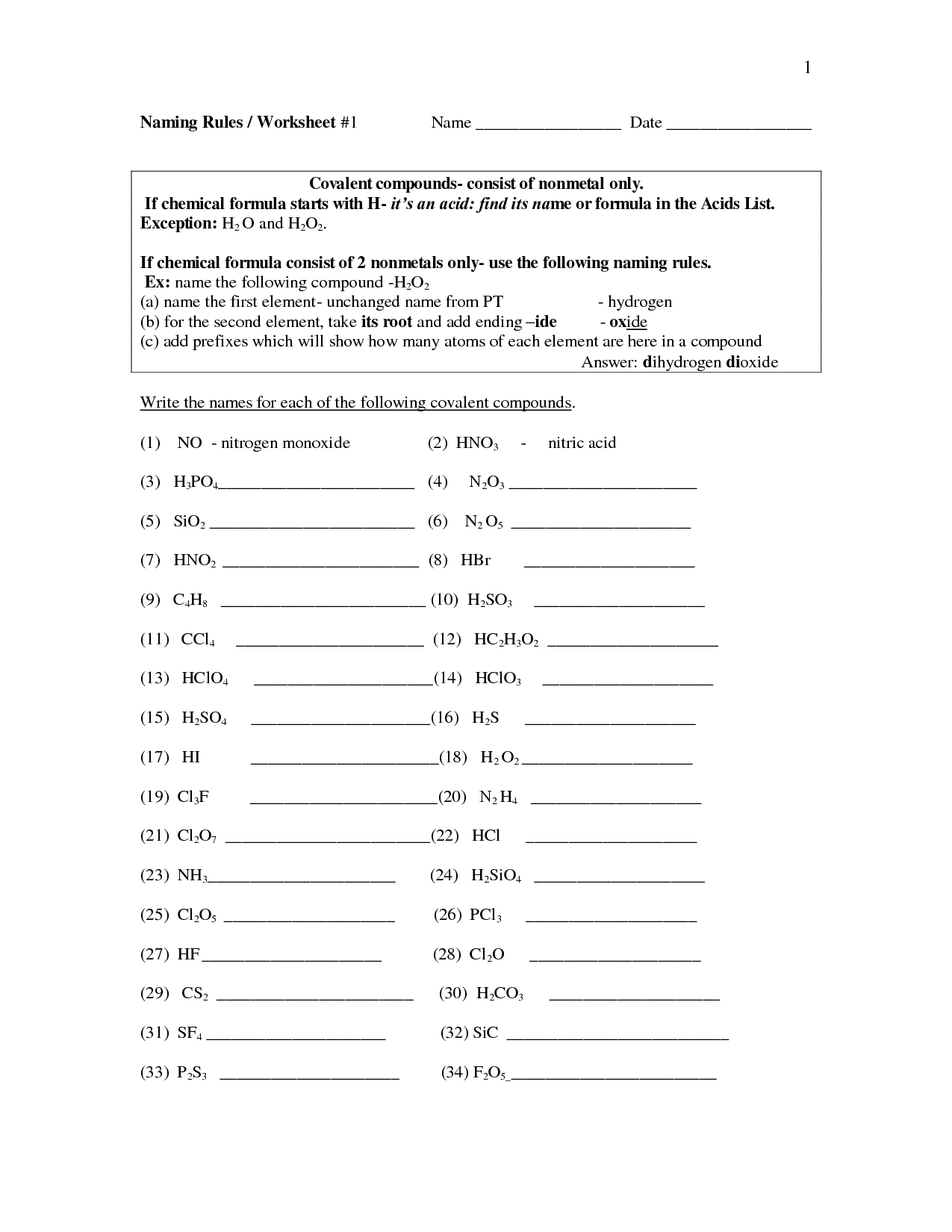
- Naming Binary Covalent Compounds Worksheet Answers
- Electron Configuration Worksheet Answer Key
- Naming Ionic Compounds Worksheet Answers
- Polyatomic Ions and Ionic Compounds
- Ionic Compounds Polyatomic Ions Worksheet Answer Key
- Atoms vs Ions Worksheet Answers
- Practice Naming Ionic Compounds Worksheet Answers
- Bohr Atomic Model Worksheet Middle School
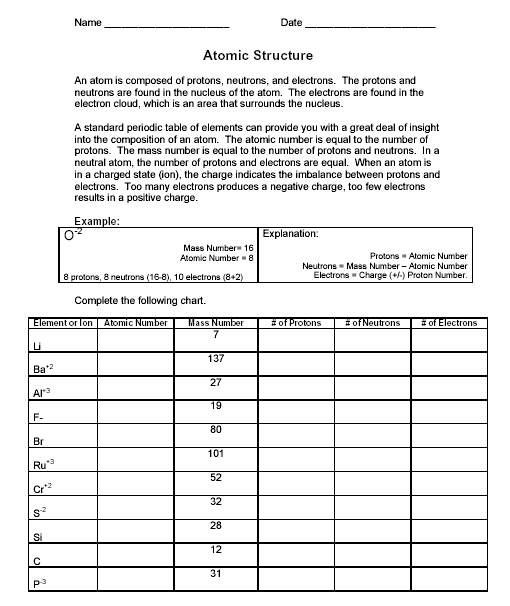
- Chemistry Atomic Structure Worksheet Answers
- Chemistry Periodic Trends Worksheet Answers
- Atoms Worksheet Answers Crossword Puzzle
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is an ion?
An ion is an atom or molecule that has gained or lost one or more electrons, giving it a positive or negative electrical charge. This charge imbalance results in the ion being capable of forming chemical bonds with other ions or molecules.
How do ions form?
Ions form when atoms gain or lose electrons. Atoms become positively charged ions (cations) when they lose electrons, leaving them with more protons than electrons. Conversely, atoms become negatively charged ions (anions) when they gain electrons, resulting in more electrons than protons. This process is driven by the tendency of atoms to achieve a stable electron configuration, typically following the octet rule, in which the outermost energy level is filled with eight electrons.
What is the difference between a cation and an anion?
A cation is a positively charged ion that forms when an atom loses one or more electrons, while an anion is a negatively charged ion that forms when an atom gains one or more electrons. In other words, cations have more protons than electrons, resulting in a net positive charge, whereas anions have more electrons than protons, giving them a net negative charge.
How do ions interact with each other?
Ions interact with each other through electrostatic forces due to their opposite charges. Positively charged ions (cations) are attracted to negatively charged ions (anions) and form ionic bonds. These bonds are strong and are responsible for holding the ions together in ionic compounds. The strength of the interaction between ions depends on the magnitude of their charges and the distance between them.
How are ions involved in chemical reactions?
Ions are involved in chemical reactions by participating as reactants or products in the formation of new substances. When ionic compounds dissolve in water or react with each other, ions are released and can interact with other ions or molecules to form new compounds. Ions can also facilitate chemical reactions by increasing the rate of reaction or by serving as catalysts. Their charges and interactions with other ions play a crucial role in determining the outcome of various chemical reactions.
What are the properties of ions in terms of solubility?
Ions can have different solubilities depending on their charge and size. Generally, ions formed by alkali metals and ammonium are soluble in water, while ions formed by halides, sulfates, and nitrates are also usually soluble. However, ions of silver, lead, and mercury are often insoluble. Additionally, the solubility of ions can be influenced by factors such as pH, temperature, and the presence of other ions in the solution.
How do ions contribute to electrical conductivity?
Ions contribute to electrical conductivity by carrying an electric charge and enabling the flow of electric current in a solution or material. In an aqueous solution, ions such as sodium (Na+) and chloride (Cl-) are free to move and carry charge, facilitating the flow of electricity. In solid materials, ions can also contribute to conductivity by allowing the movement of charges through a lattice structure. Overall, the presence and movement of ions play a crucial role in determining the electrical conductivity of a substance.
How are ions used in various industrial processes?
Ions are used in various industrial processes such as water treatment to remove contaminants through ion-exchange processes and in electroplating to deposit metal ions onto surfaces. In the pharmaceutical industry, ions are used in the formulation of drugs and in the production of batteries and fuel cells. Additionally, ions are employed in the manufacturing of semiconductors, ceramics, textiles, and in the food industry for processes like fermentation and preservation. Overall, ions play a crucial role in multiple industrial applications due to their ability to facilitate chemical reactions, conduct electricity, and control the properties of materials.
What is the role of ions in biological systems?
Ions play a crucial role in biological systems by helping to regulate various physiological processes, such as nerve transmission, muscle contraction, and cell signaling. They help maintain the balance of charges within cells, which is essential for the functioning of enzymes and other proteins. Additionally, ions are involved in the movement of nutrients and waste products across cell membranes, as well as in the regulation of water balance and pH levels. Overall, ions are essential for the proper functioning of cells and are critical for life processes in organisms.
How are ions detected and analyzed in laboratory settings?
Ions can be detected and analyzed in laboratory settings using various techniques such as mass spectrometry, ion chromatography, ion-selective electrodes, and atomic absorption spectroscopy. Mass spectrometry identifies ions based on their mass-to-charge ratio, while ion chromatography separates ions based on their interactions with a stationary phase. Ion-selective electrodes measure ion concentration based on the potential difference generated when the electrode comes into contact with the ion of interest. Atomic absorption spectroscopy works by measuring the absorption of light by ions in a sample, allowing for quantification of their concentration.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments