Introduction to Periodic Table Worksheet
The Periodic Table is a fundamental tool for understanding the elements and their properties. To help students grasp the concepts behind this important scientific resource, worksheets can provide a valuable learning tool. By exploring various worksheets designed around the Periodic Table, students can strengthen their understanding of element names, symbols, atomic numbers, and other key information. With a wide range of options available, there is sure to be a worksheet suitable for every learner looking to enhance their knowledge of the Periodic Table.
Table of Images 👆
- AP Chemistry Worksheet Lewis Structure
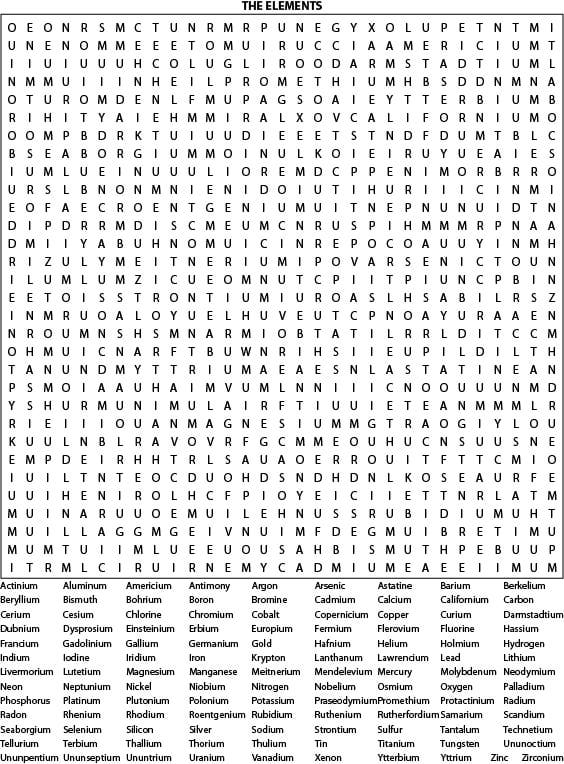
- Chemistry Element Word Search Answers
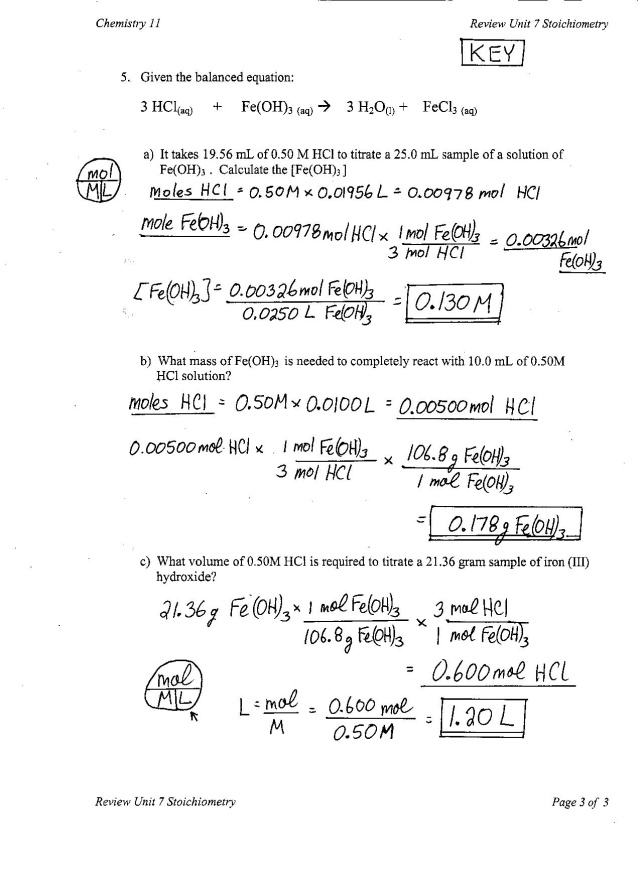
- Extra Practice Answers Chapter 7
- Crossword Puzzle Answer Key
- Calorimetry Lab Specific Heat
- Chapter 25 Nuclear Chemistry Answer Key
- Chemical Reaction Types Worksheet
- Modeling Chemistry Worksheet Answer Key
- Quantitative Worksheets More Less
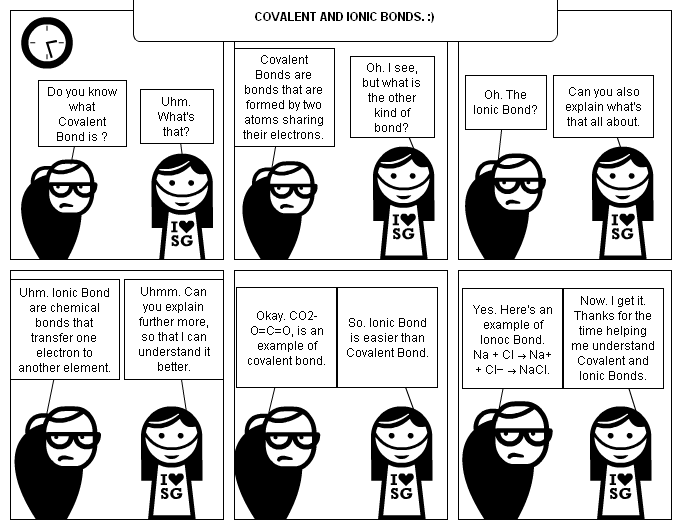
- Ionic and Covalent Bond Comic Strip
- DNA the Molecule of Heredity Worksheet Answer Key
- DNA the Molecule of Heredity Worksheet Answer Key
- DNA the Molecule of Heredity Worksheet Answer Key
- DNA the Molecule of Heredity Worksheet Answer Key
- DNA the Molecule of Heredity Worksheet Answer Key
- DNA the Molecule of Heredity Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the periodic table?
The periodic table is a tabular arrangement of the chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. It allows elements to be classified by their similar characteristics and trends in their properties. Created by Dmitri Mendeleev in 1869, the periodic table is one of the most important tools in chemistry for understanding the relationships between different elements and predicting their behaviors.
Who is credited with creating the first periodic table?
The first periodic table was created by Dmitri Mendeleev, a Russian chemist, in 1869. Mendeleev arranged the elements in order of increasing atomic weight and grouped them based on their chemical properties, predicting the existence and properties of several yet-to-be-discovered elements.
How is the periodic table organized?
The periodic table is organized by increasing atomic number from left to right and top to bottom. Elements are classified into periods (rows) and groups (columns) based on similar chemical properties. The table is divided into metals, non-metals, and metalloids, with elements arranged in groups such as alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. The structure allows scientists to predict properties and behaviors of elements based on their position in the table.
What are periods and groups in the periodic table?
Periods in the periodic table refer to the horizontal rows of elements, numbered from 1 to 7, that indicate the number of electron shells an atom has. Groups, on the other hand, are the vertical columns of elements that share similar chemical properties due to having the same number of valence electrons. Each group is numbered from 1 to 18, with the elements within a group having similar characteristics and reactivity.
How many elements are currently known and listed in the periodic table?
There are currently 118 elements known and listed in the periodic table.
How are elements arranged within a group or family?
Elements within a group or family are arranged in order of increasing atomic number, which reflects the number of protons in the nucleus of each atom. The group/family number is determined by the number of valence electrons an element has, which influences its chemical properties. Elements within the same group/family share similar chemical characteristics due to their similar electronic configurations and the way they interact with other elements. Additionally, elements in the same group often form compounds with similar properties, making it easier to predict their behavior based on their placement in the periodic table.
How are elements arranged within a period or series?
Elements within a period or series are arranged in increasing order of atomic number. This means that elements are placed in order of the number of protons in the nucleus of their atoms. As you move from left to right across a period in the periodic table, the number of protons increases by one, resulting in chemical properties that change gradually.
What information can be found for each element in the periodic table?
For each element in the periodic table, you can typically find information such as its atomic number, atomic mass, symbol, group, period, name, electron configuration, physical properties like melting and boiling points, density, and atomic radius, as well as chemical properties like reactivity and common compounds formed. Additionally, details on its history, discovery, uses, isotopes, and any special characteristics or applications of the element may also be included in the information provided.
How are elements classified in terms of their properties on the periodic table?
Elements on the periodic table are classified based on their properties such as atomic number, atomic mass, electron configuration, and chemical reactivity. They are grouped into periods (rows) and groups (columns) according to shared characteristics. Elements in the same period have similar electron configurations, while elements in the same group have similar chemical properties due to having the same number of valence electrons. This classification helps organize elements and predict their behaviors based on their placement within the periodic table.
What is the significance of the periodic table in understanding the behavior and relationships of elements?
The periodic table is significant in understanding the behavior and relationships of elements because it organizes elements based on their atomic number, electron configuration, and chemical properties. By grouping elements with similar characteristics together in columns, called groups or families, we can predict their behavior, such as reactivity, atomic size, and chemical bonding. This organization allows scientists to easily identify trends and patterns in element properties, leading to a better understanding of the fundamental principles underlying chemistry and the natural world.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments