Elements Compounds and Mixtures Worksheet Answer Key
Worksheets are an invaluable resource for students who are seeking a deeper understanding of the concept of elements, compounds, and mixtures. Designed to enhance learning, this entity provides a clear and concise answer key that allows students to reinforce their understanding of this subject matter through practice and reflection. By utilizing this worksheet, students can confidently tackle challenging questions and strengthen their knowledge in a structured and independent manner.
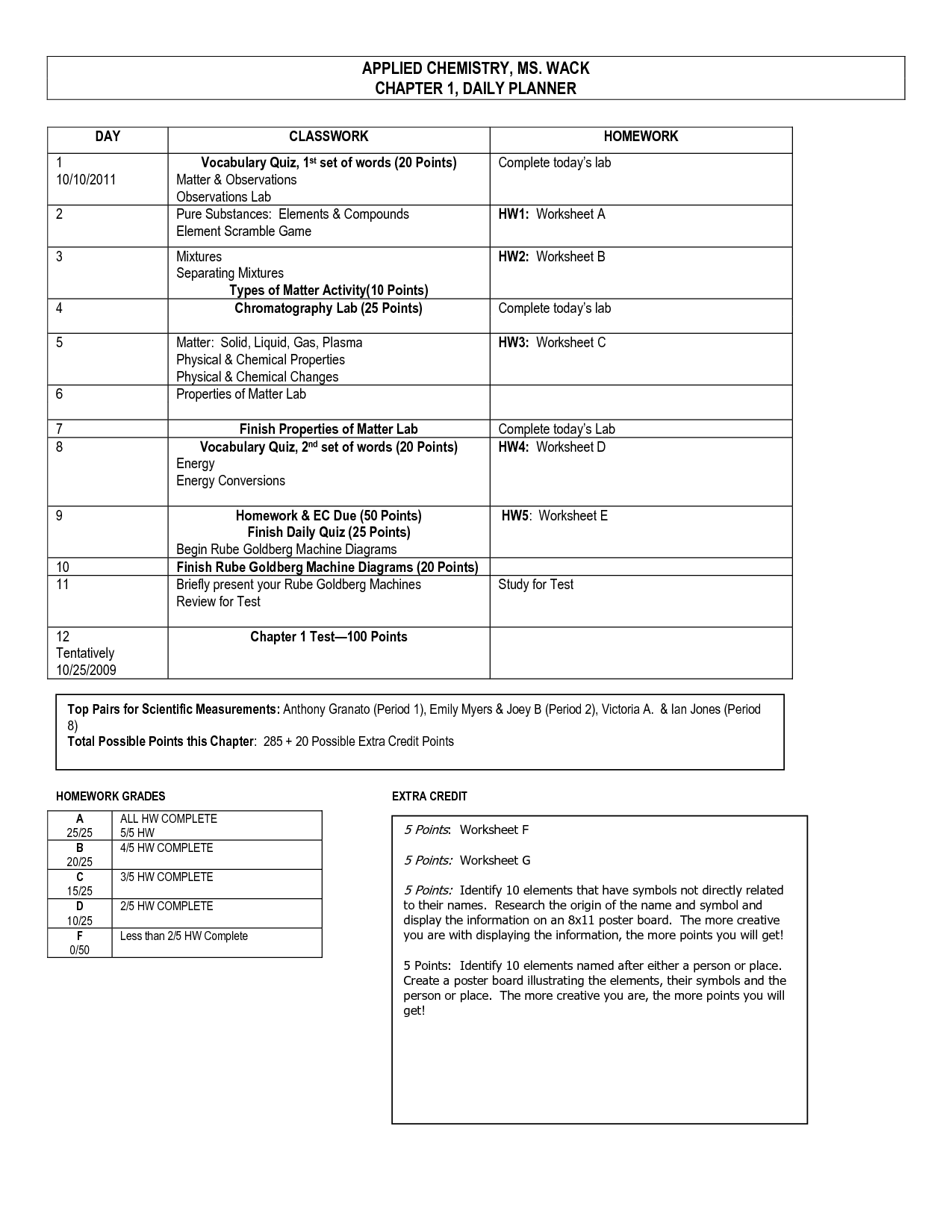
Table of Images 👆
- Element Compound Mixture Worksheet
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
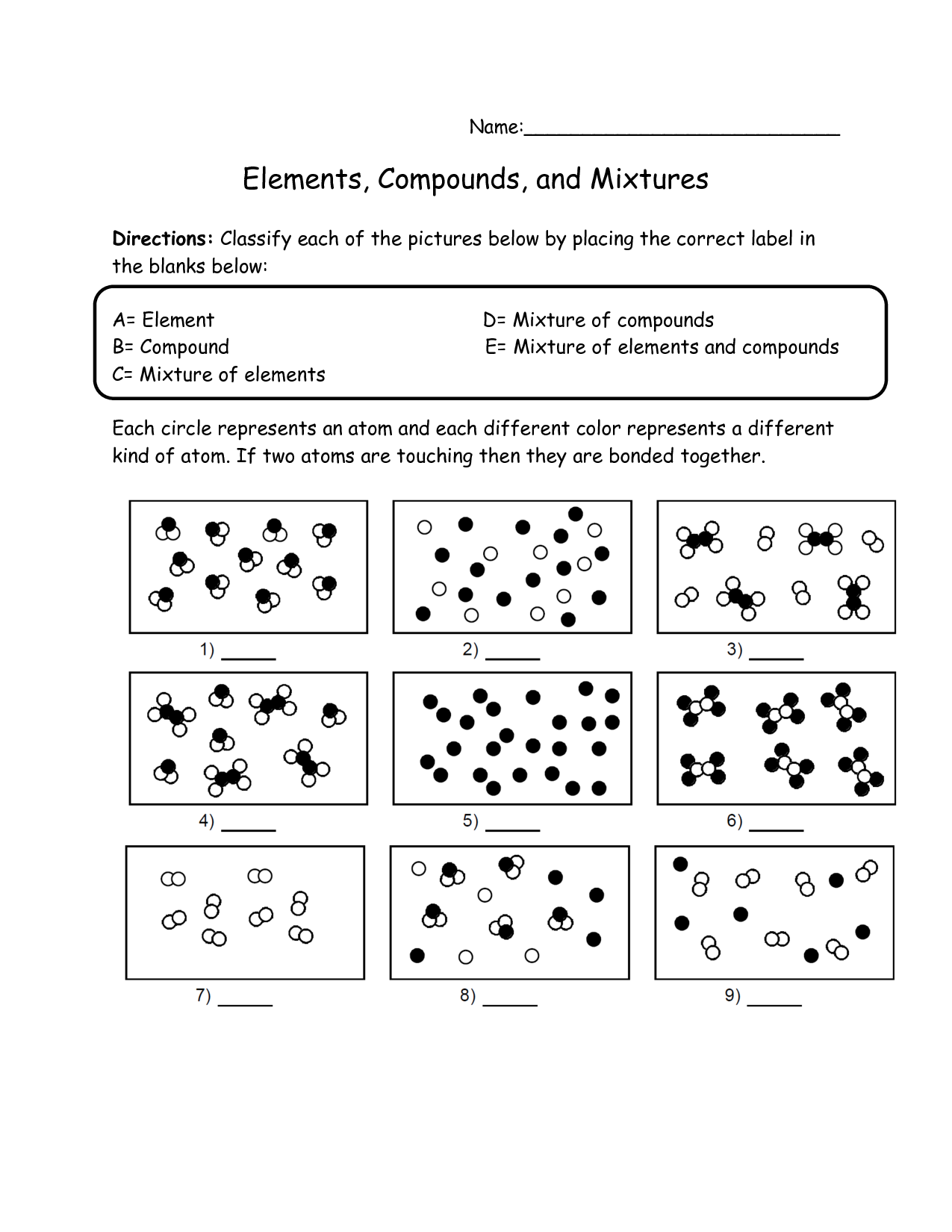
- Elements Compounds and Mixtures Worksheet Key
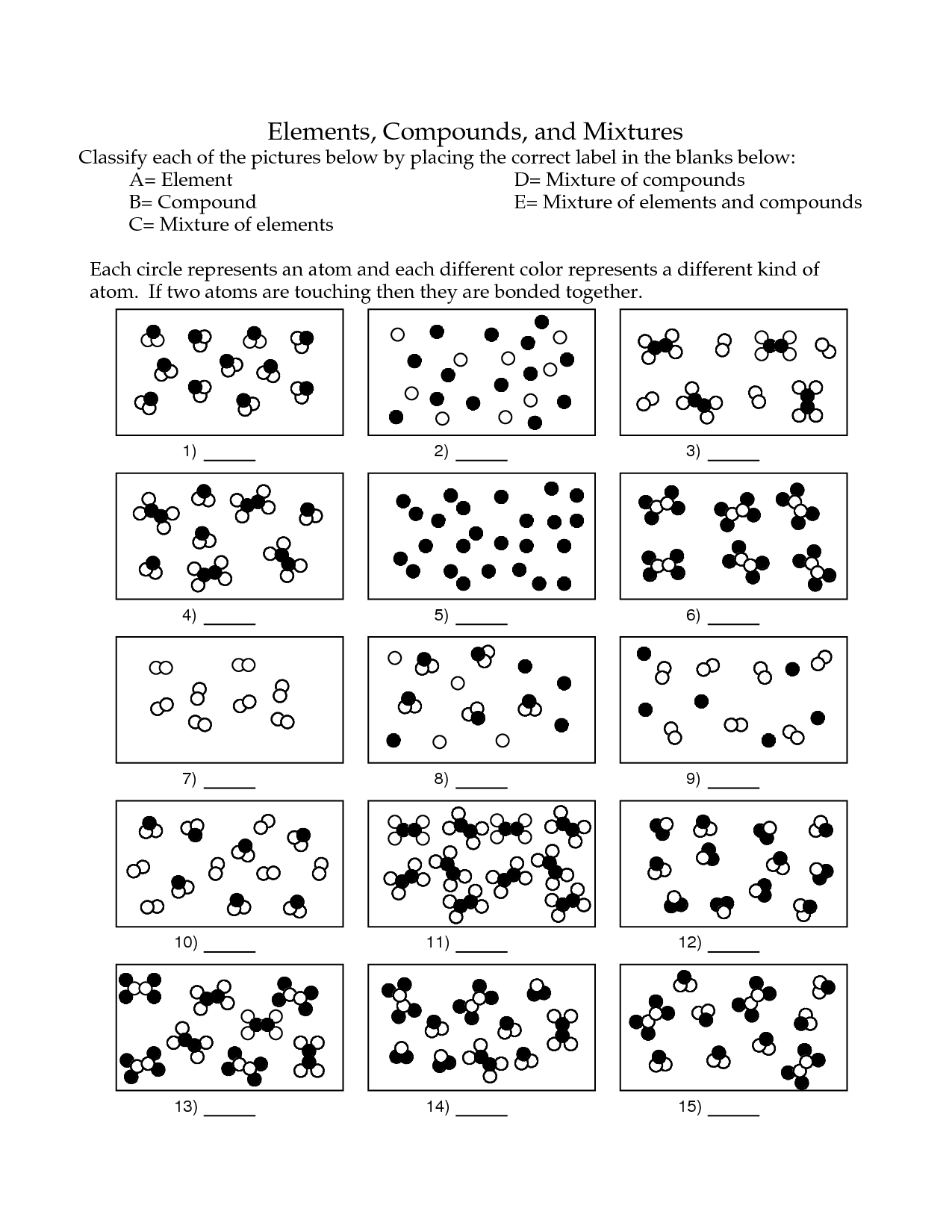
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Counting Atoms in Compounds Worksheet Answers
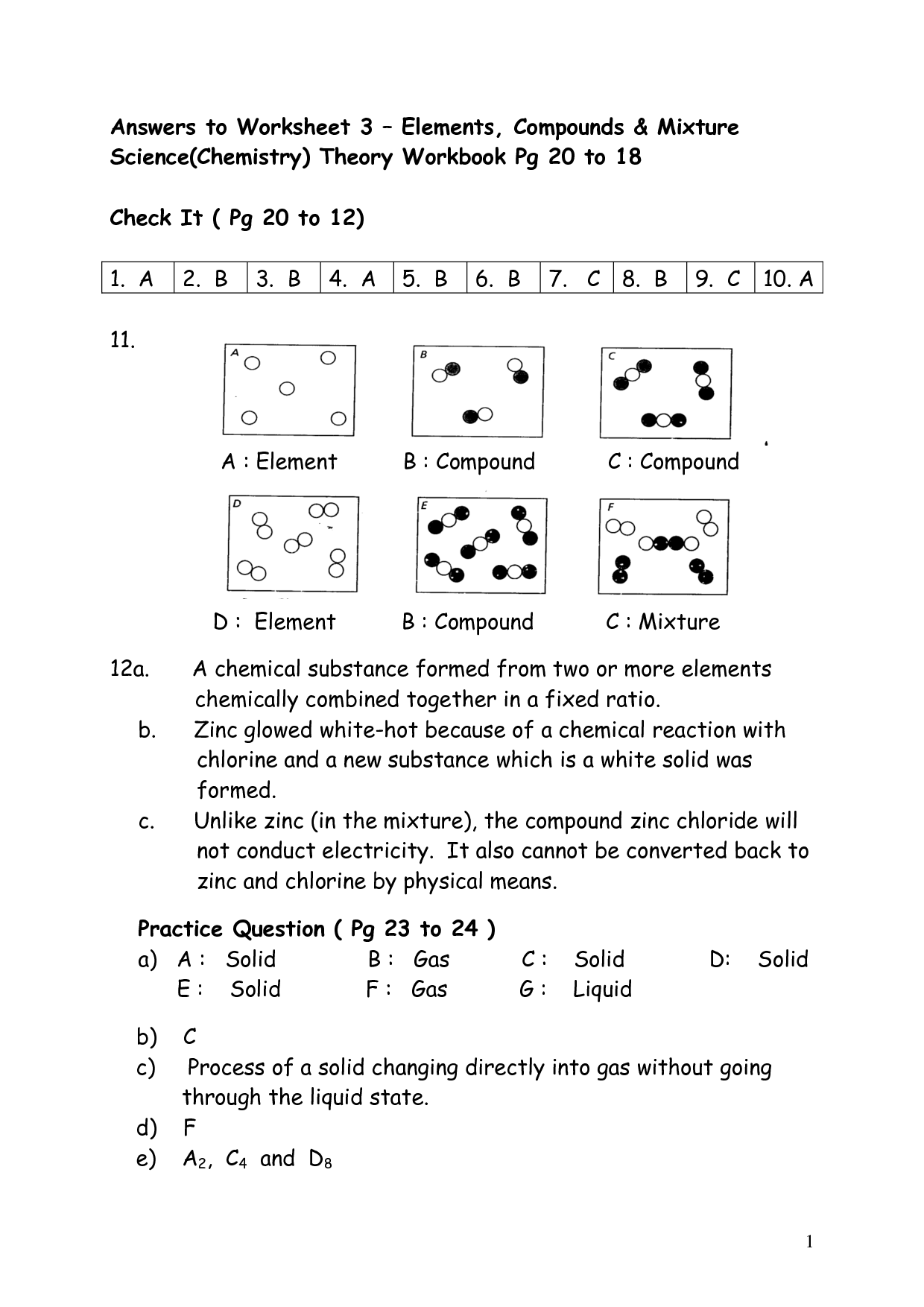
- Elements Compounds and Mixtures Answer Key
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Naming Ionic Compounds Worksheet Answer Key
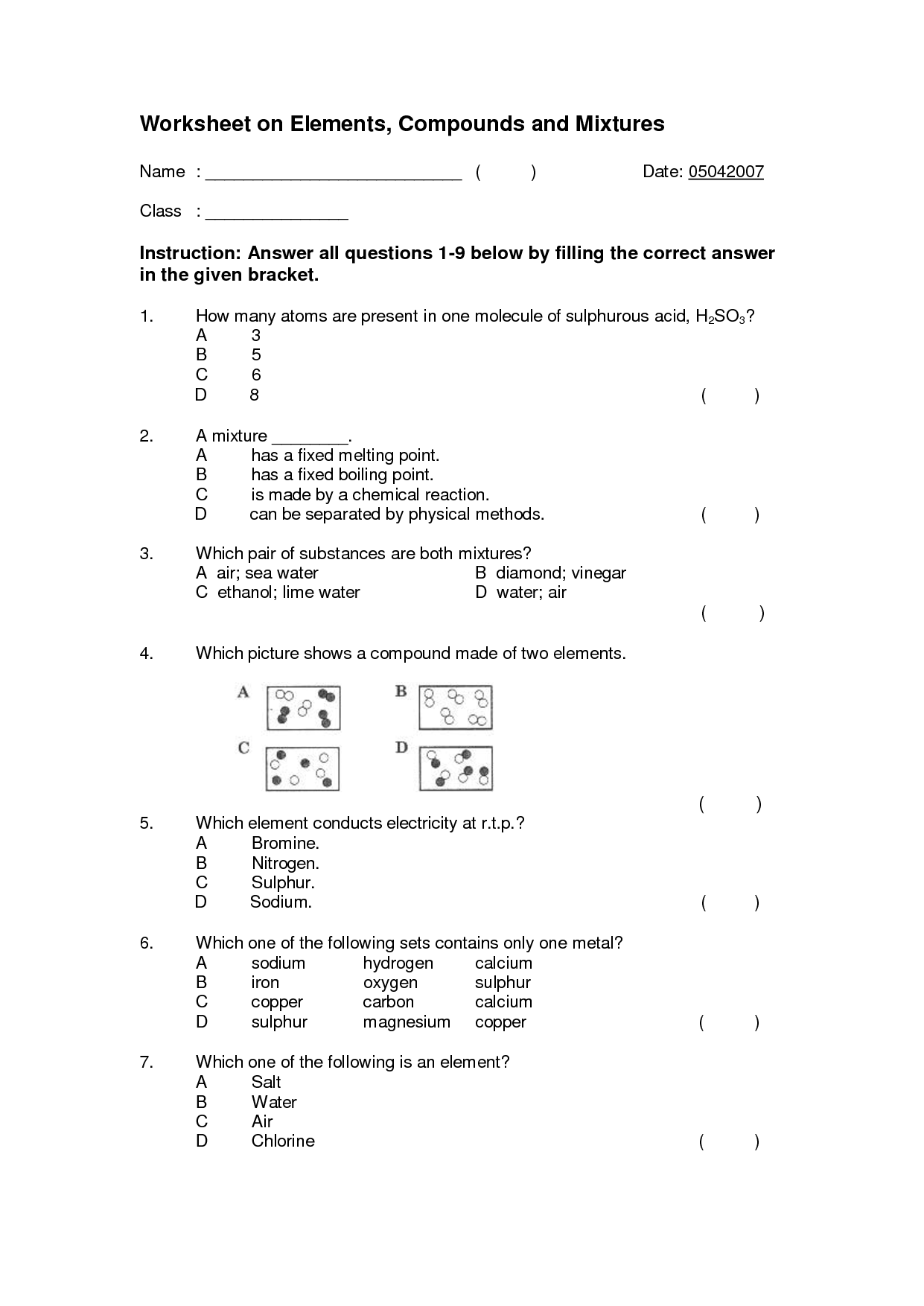
- Elements Compounds and Mixtures Worksheet
- Elements Compounds and Mixtures Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
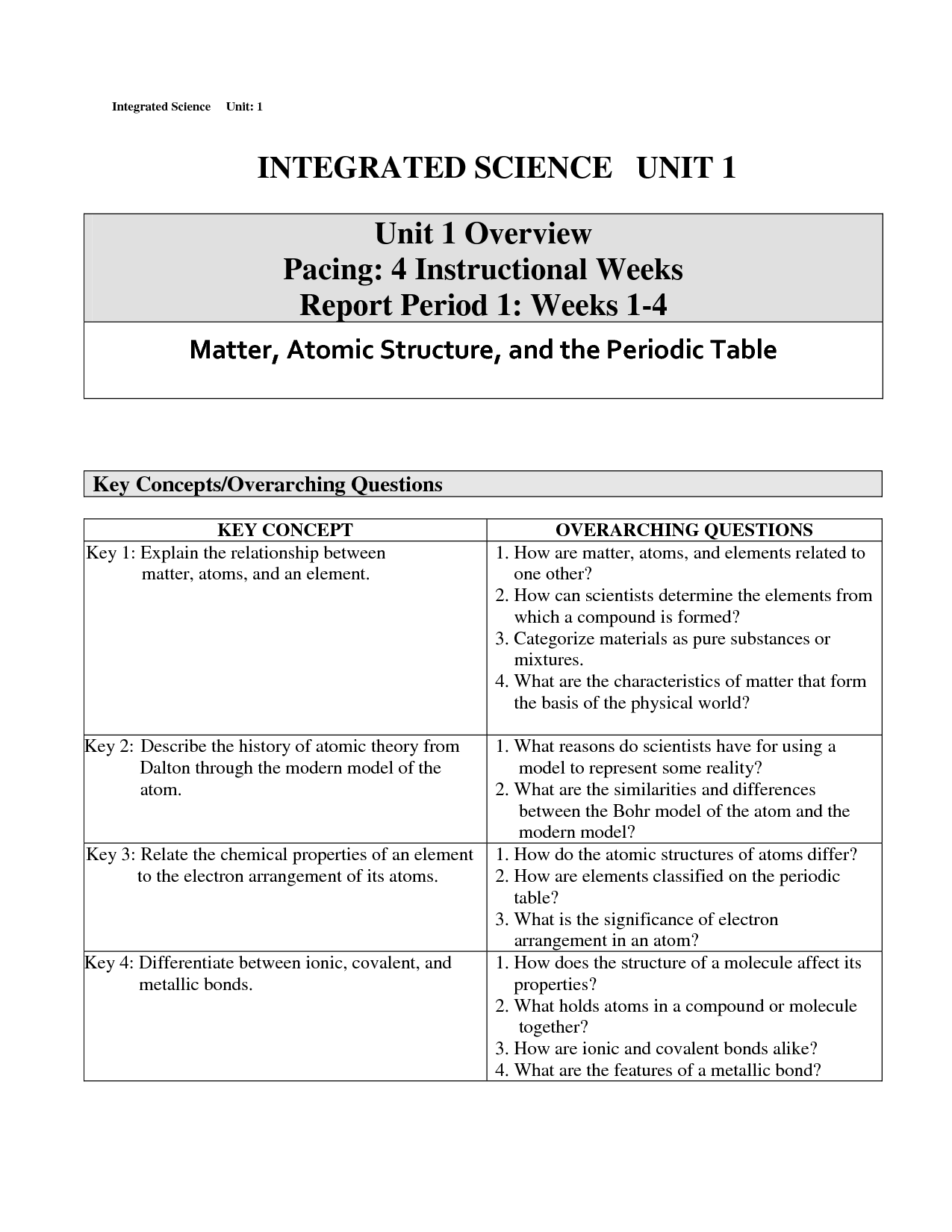
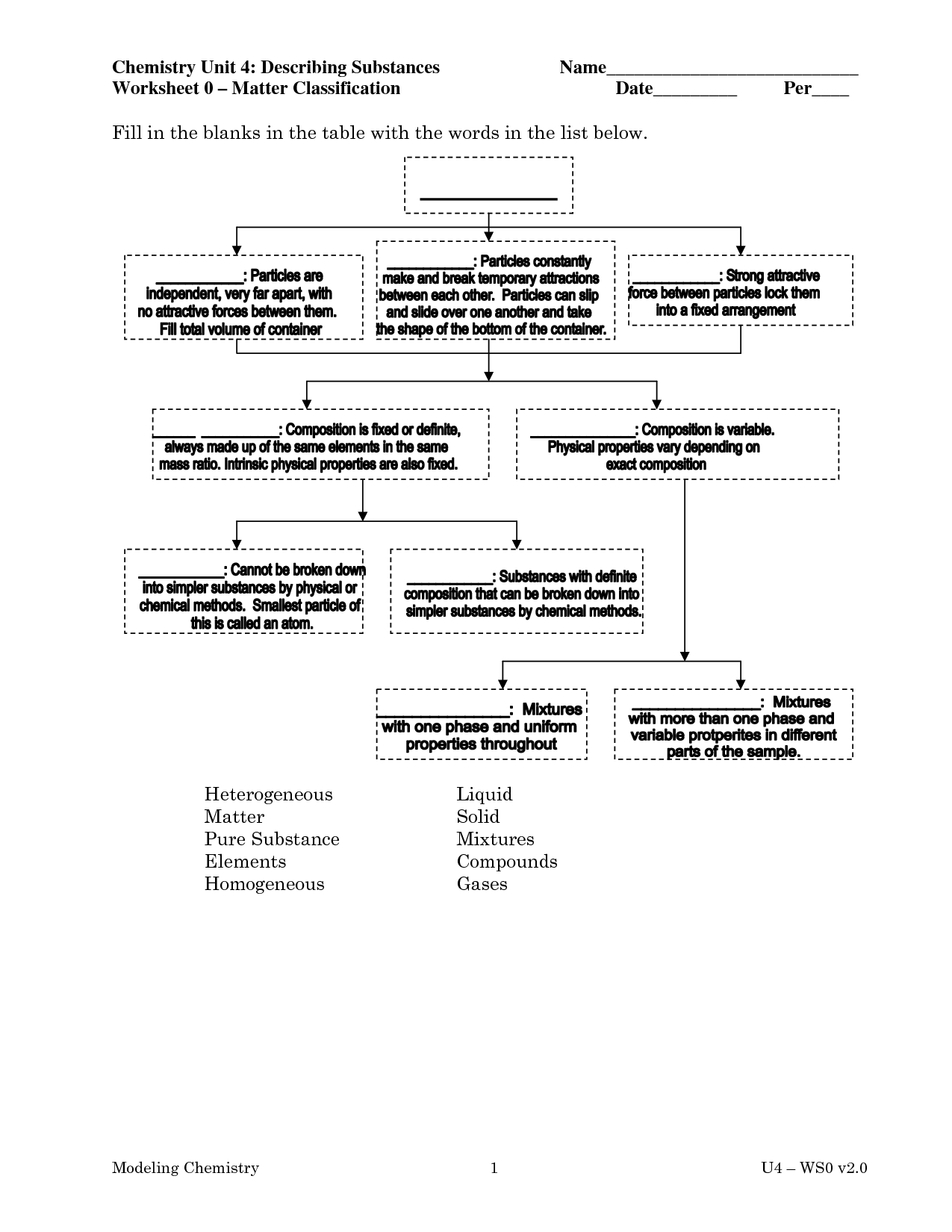
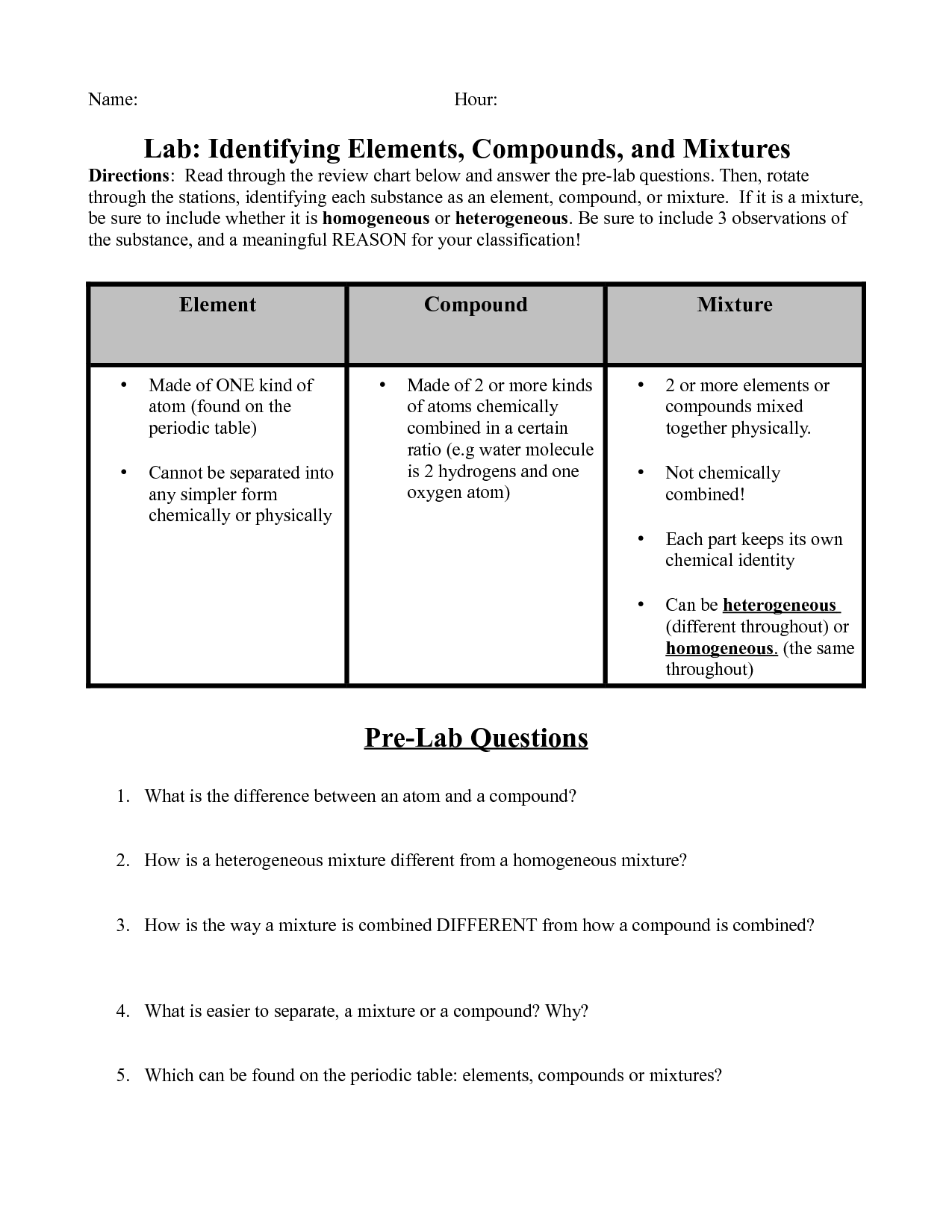
What is an element?
An element is a substance that cannot be broken down by chemical means into simpler substances. Each element consists of atoms that have the same number of protons in their nuclei. Elements are organized in the periodic table based on their unique atomic number, which is the number of protons in an atom of that element.
A substance composed of atoms that have the same number of protons in their nucleus.
The substance you are referring to is an element. Elements are composed of atoms that have the same number of protons in their nucleus, which determines their unique chemical properties and identity on the periodic table.
Describe a compound.
A compound is a substance composed of two or more different elements chemically bonded together in specific proportions. Compounds have unique properties that are different from the properties of the individual elements that make them up. Examples of compounds include water (H2O), sodium chloride (NaCl), and carbon dioxide (CO2).
A substance made up of two or more different elements chemically bonded together in fixed proportions.
A compound is a substance composed of two or more different elements that are chemically bonded together in specific proportions, forming a distinct and unique substance with different properties than its constituent elements.
What is a mixture?
A mixture is a combination of two or more substances that are physically intermingled but do not chemically react with each other. Mixtures can be homogeneous, where the components are evenly distributed, or heterogeneous, where the components are unevenly distributed.
A combination of two or more substances that are physically mixed together but not chemically bonded.
The combination of two or more substances that are physically mixed together but not chemically bonded is called a mixture. Mixtures can be separated through physical means, such as filtration or distillation, due to the lack of chemical bonds holding the components together.
Explain the difference between a homogeneous mixture and a heterogeneous mixture.
A homogeneous mixture has uniform composition throughout, with all its components evenly distributed, such as salt dissolved in water. In contrast, a heterogeneous mixture has non-uniform composition, with its components not evenly distributed, like a salad with different vegetables mixed together.
A homogeneous mixture has a uniform composition throughout, while a heterogeneous mixture has an uneven distribution of substances.
In a homogeneous mixture, the components are evenly distributed and have a uniform composition, making it challenging to distinguish between the individual substances, whereas in a heterogeneous mixture, the components are not uniformly distributed and can be easily identified by physical separation methods due to their uneven distribution throughout the mixture.
Name an example of an element.
Gold is an example of an element.
Oxygen (O), found in the air.
Oxygen (O) is a vital element found in the Earth's atmosphere, making up about 21% of the air we breathe. It is crucial for the survival of most living organisms, as it is utilized in cellular respiration to produce energy and for various metabolic processes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments