Density Practice Worksheet
Are you a student or teacher in need of additional practice with density calculations? Look no further! This density practice worksheet is designed to help you solidify your understanding of this fundamental concept in physical science. Whether you're just starting to learn about density or need a refresher, this worksheet will provide you with ample opportunity to practice calculating density and applying the formula.
Table of Images 👆
- Mole Calculation Worksheet Answer Key
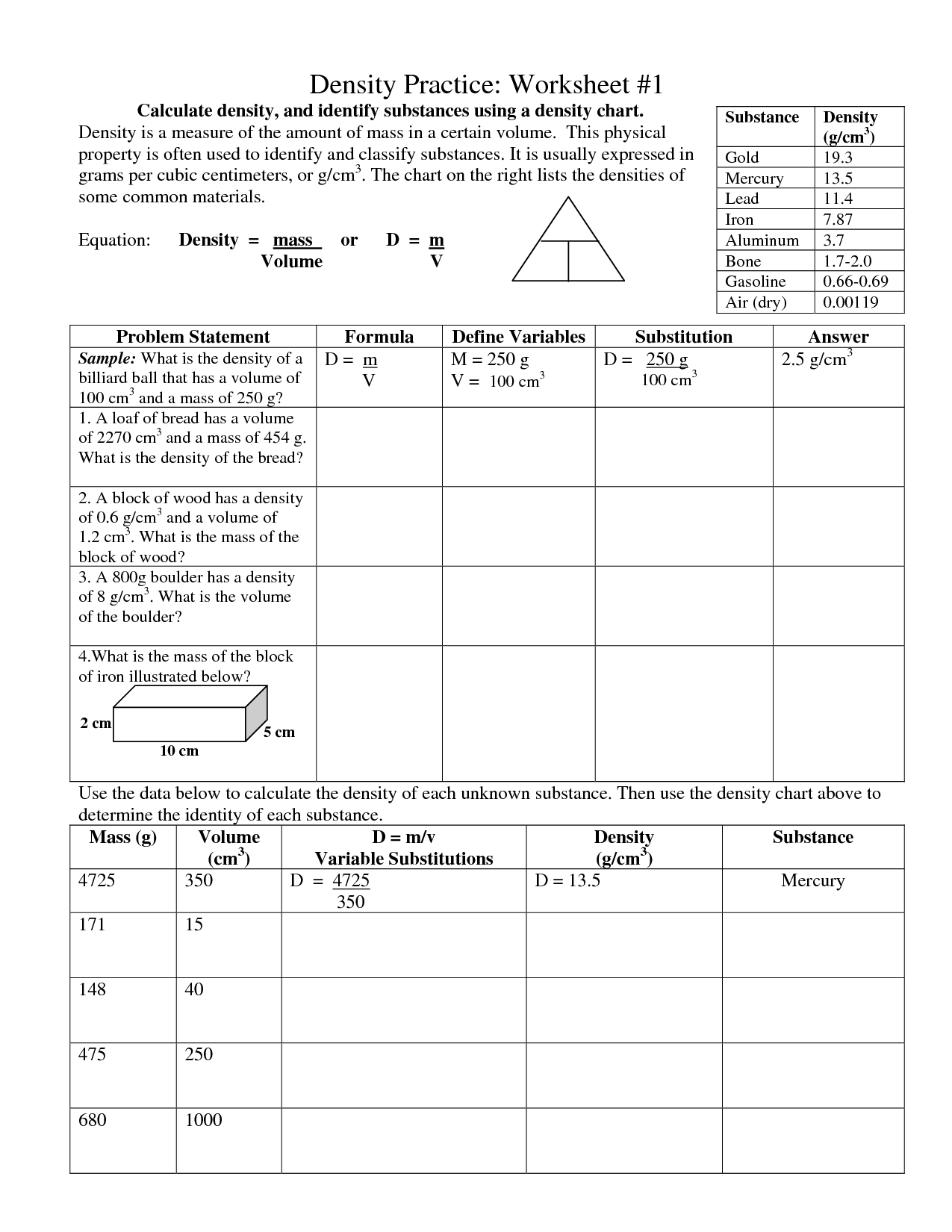
- Density Practice Worksheet 1
- Significant Figures Worksheet and Answers
- Parallel Circuit Problem Worksheet
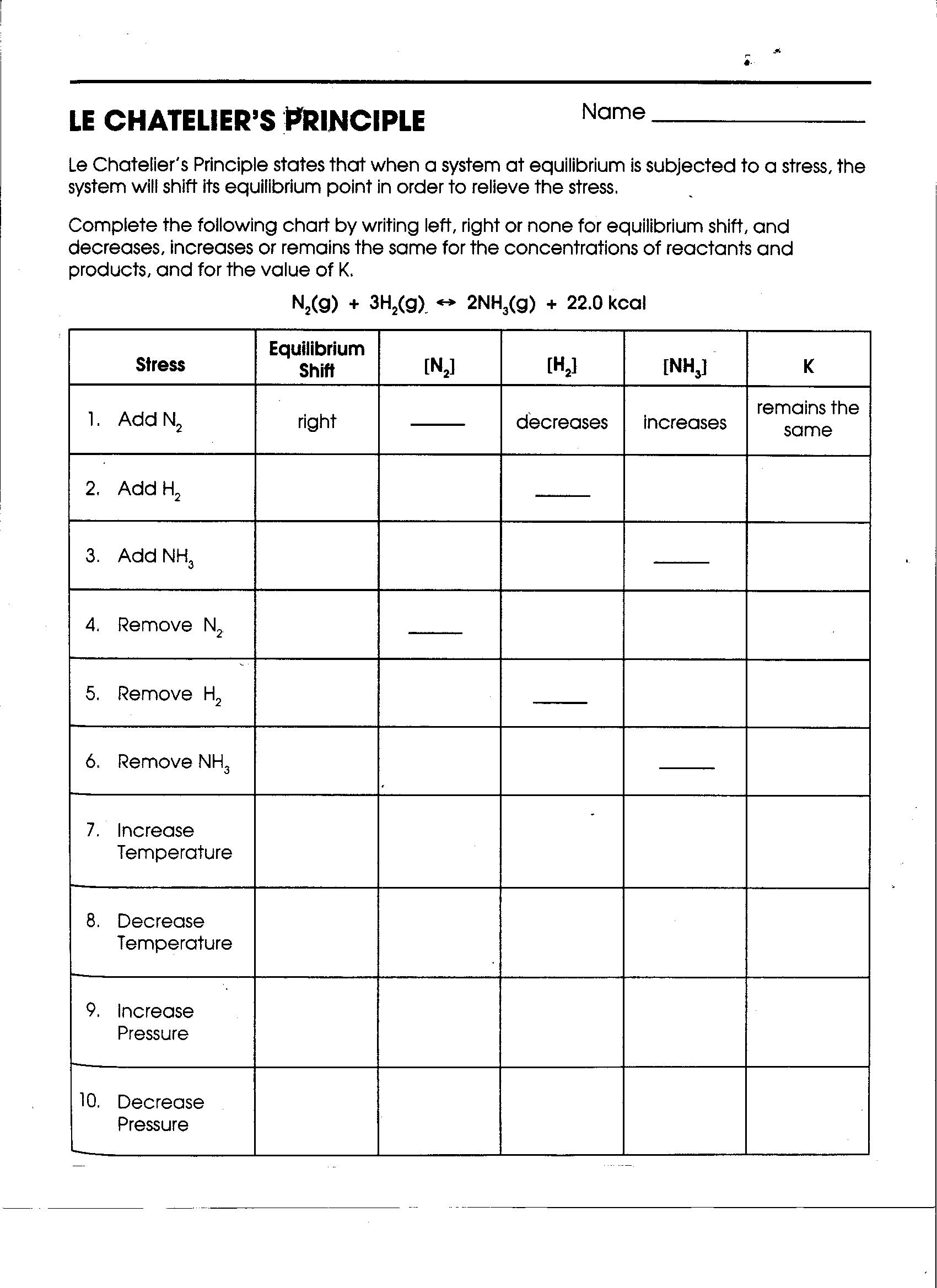
- Le Chateliers Principle Chart
- 4th Grade Science Worksheets
- Triple Beam Balance Worksheet
- Worksheets Answer Key
- Density Equation Worksheet
- VSEPR Molecular Geometry Chart
- Atomic Structure Worksheet Answer Key
- Bill Nye Chemical Reactions Worksheet Answers
- Science Skills Worksheets Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is density?
Density is a physical property that measures how much mass is present in a given volume of a substance. It is calculated by dividing the mass of an object by its volume. Objects with higher density have more mass packed into a smaller space, while objects with lower density have less mass spread out over a larger space. Density is commonly expressed in units such as grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L).
How is density calculated?
Density is calculated by dividing the mass of an object by its volume. The formula for density is: Density = mass / volume. The unit of density is typically expressed in grams per cubic centimeter (g/cm^3) for solids and liquids, or kilograms per cubic meter (kg/m^3) for gases.
What are the units of density commonly used?
The most commonly used units for density are grams per cubic centimeter (g/cm³) and kilograms per cubic meter (kg/m³).
How does the density of an object affect its buoyancy?
The density of an object directly affects its buoyancy, with less dense objects floating higher in a fluid compared to more dense objects. This is due to the principle of buoyancy, which states that the buoyant force acting on an object in a fluid is equal to the weight of the fluid displaced by the object. Therefore, objects with lower density displace more fluid and experience a greater buoyant force, causing them to float or rise in the fluid, while objects with higher density displace less fluid and sink or remain lower in the fluid.
How does the density of a substance determine whether it will float or sink in a liquid?
The density of a substance determines whether it will float or sink in a liquid based on the concept of buoyancy. If the density of the substance is greater than the density of the liquid, it will sink. However, if the density of the substance is less than the density of the liquid, it will float. This is because objects with higher density than the liquid will displace less liquid and experience a greater gravitational force pulling them down, causing them to sink. Conversely, objects with lower density than the liquid will displace more liquid and experience an upwards buoyant force, causing them to float.
What is the relationship between mass, volume, and density?
Density is calculated by dividing an object's mass by its volume. This means that mass and volume are related to density by the equation density = mass / volume. Generally, as the mass of an object increases while the volume remains constant, the density also increases. Conversely, if the mass is held constant and the volume increases, the density decreases.
How does the density of a material affect its heat conductivity?
The density of a material generally has a direct relationship with its heat conductivity, where higher density materials tend to have higher heat conductivity. This is because denser materials have a greater number of particles in a given volume, allowing for more collisions between particles and thus facilitating faster heat transfer. On the other hand, low-density materials have fewer particles and hence slower heat transfer.
How does temperature affect the density of a substance?
Temperature affects the density of a substance by changing the intermolecular distances within the material. As temperature increases, the thermal energy causes the molecules to vibrate more vigorously, leading to increased kinetic energy and creating more space between the molecules, thus decreasing the density of the substance. Conversely, as temperature decreases, the molecules become less agitated, moving closer together and increasing the density of the substance.
How does pressure affect the density of a gas?
Pressure and density of a gas are directly related. As pressure increases on a gas, the gas molecules are pushed closer together, which decreases the volume of space between them. This results in an increase in the gas density as more gas molecules are now contained in a smaller volume. Conversely, when pressure decreases, the gas molecules have more space to move around, leading to a decrease in density as the molecules are spread out over a larger volume.
How can the density of a mixture be determined if the densities of the individual components are known?
To determine the density of a mixture when the densities of the individual components are known, you can use the mass and volume ratios of each component in the mixture. By multiplying the density of each component by its respective mass fraction and summing up these values, you can calculate the overall density of the mixture. This formula takes into account the contributions of each component based on their densities and proportions in the mixture.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments