Classification of Matter Worksheet
If you're searching for a reliable resource to deepen your understanding of the classification of matter, this blog post is for you. In this article, we will explore worksheets that focus on entity and subject, providing a structured approach to help learners grasp this essential concept.
Table of Images 👆
- States of Matter Worksheet Answers
- Classifying Matter Worksheet Answers
- Classifying Matter Worksheet Answers
- Classifying Matter Worksheet Answers
- Classification of Matter Worksheet Answers
- Physical vs Chemical Properties Worksheet
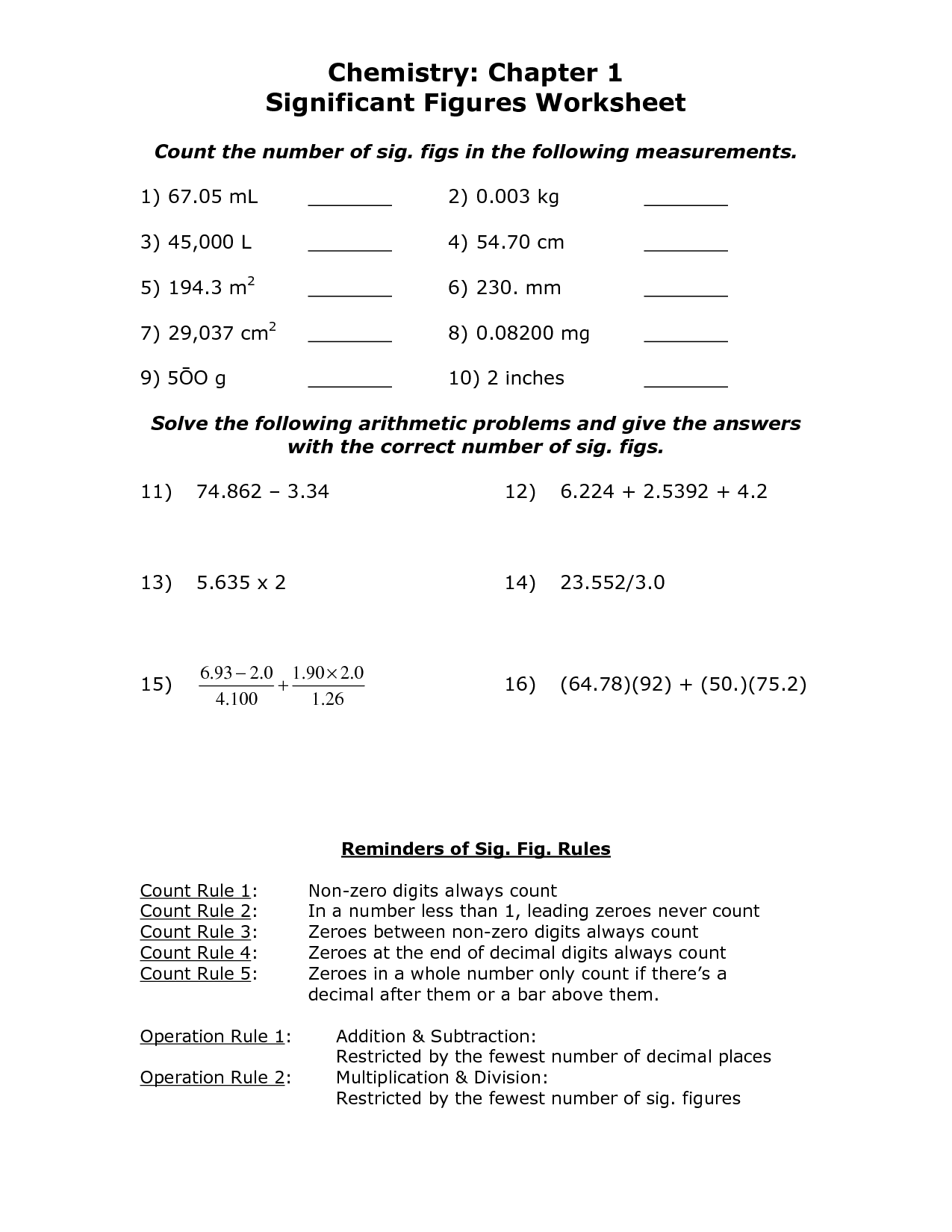
- Significant Figures Chemistry Worksheet Answers
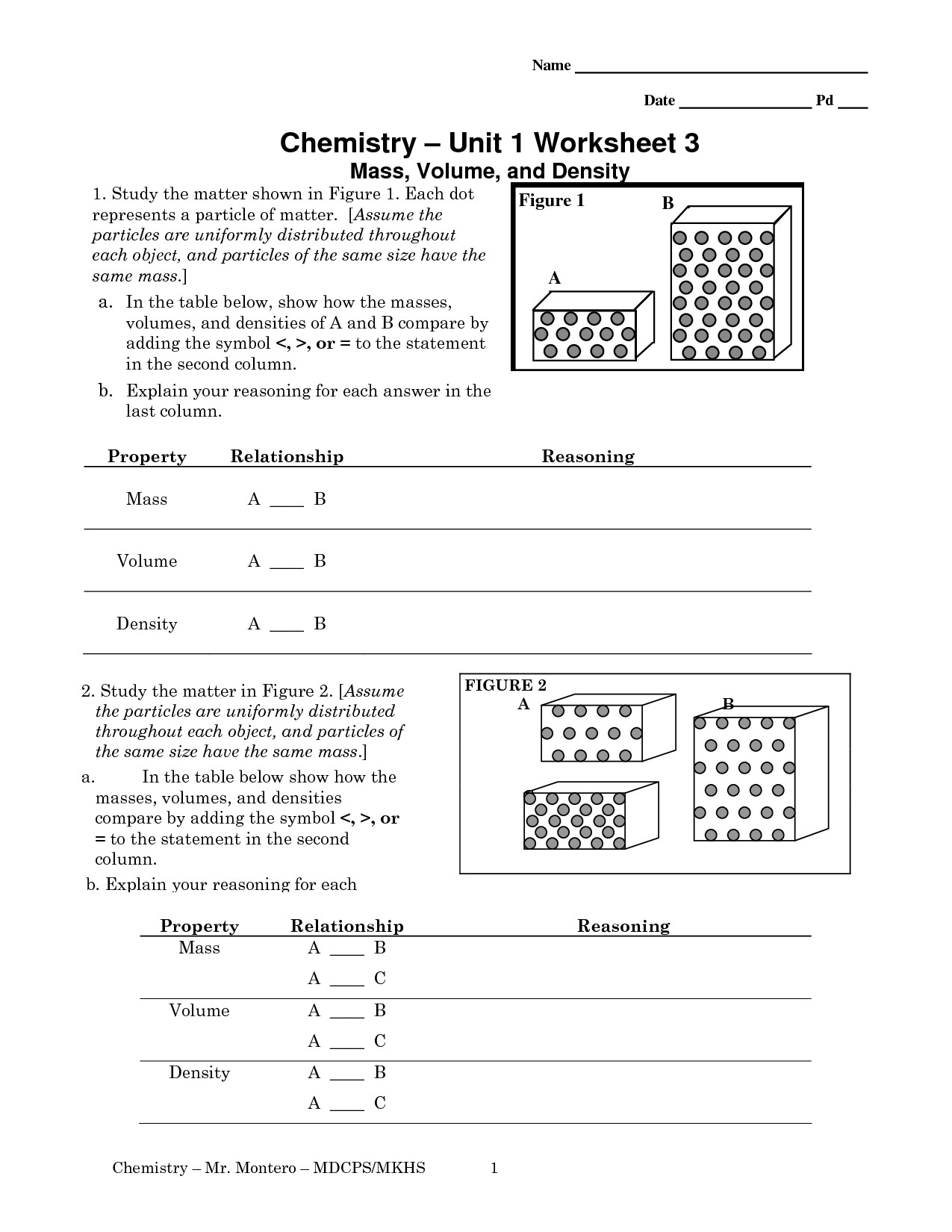
- Density Mass and Volume Worksheet
- Science States of Matter Worksheets
- Chemistry Worksheet Answer Keys
- Chemical Reactions Worksheet
- Elements and Their Symbols Worksheet
- Matter Classification Chart
- State of Matter Gas Liquid and Solids Worksheets
- Natural Resources Worksheets 3rd Grade
- Sink Float Worksheet
- Force and Motion Worksheets
- Force and Motion Worksheets
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
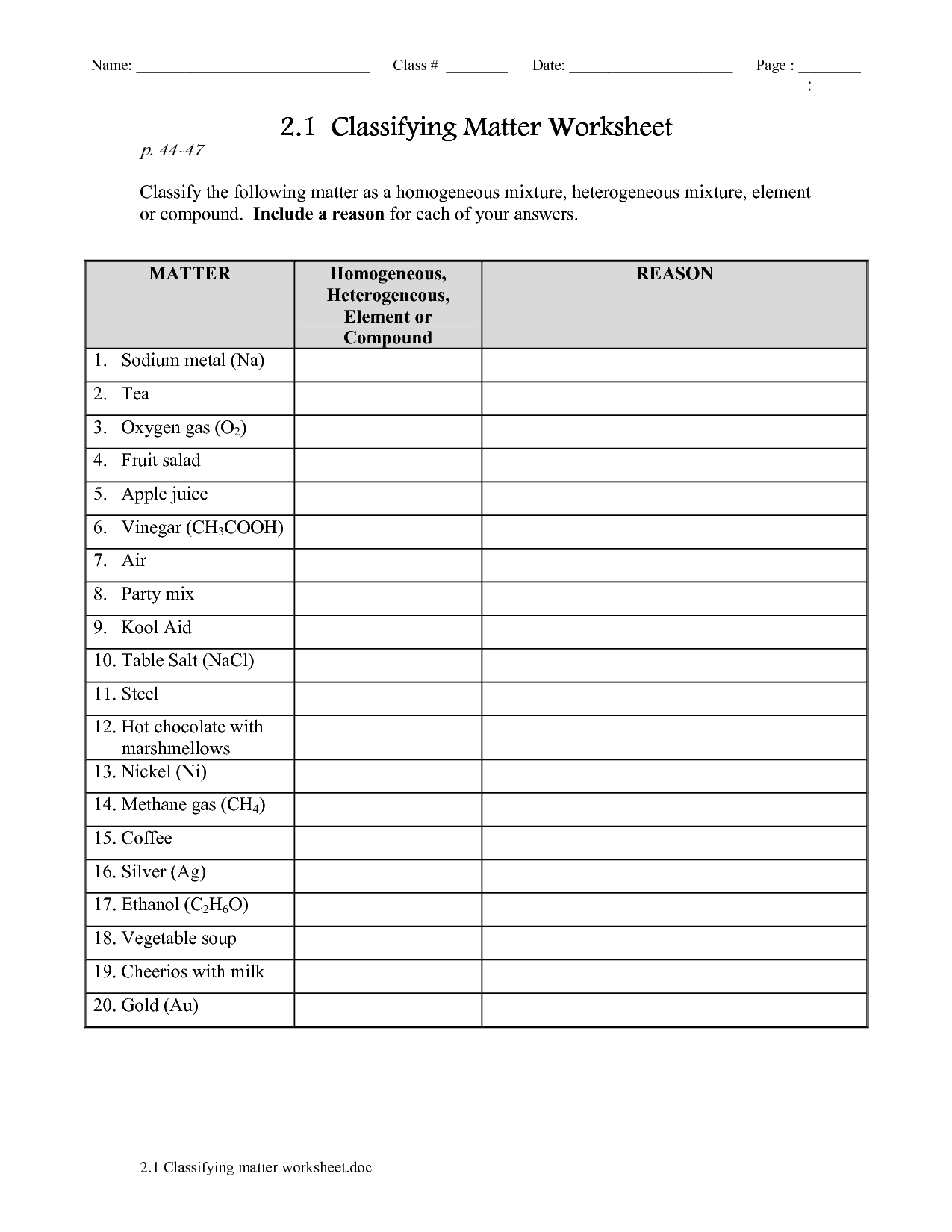
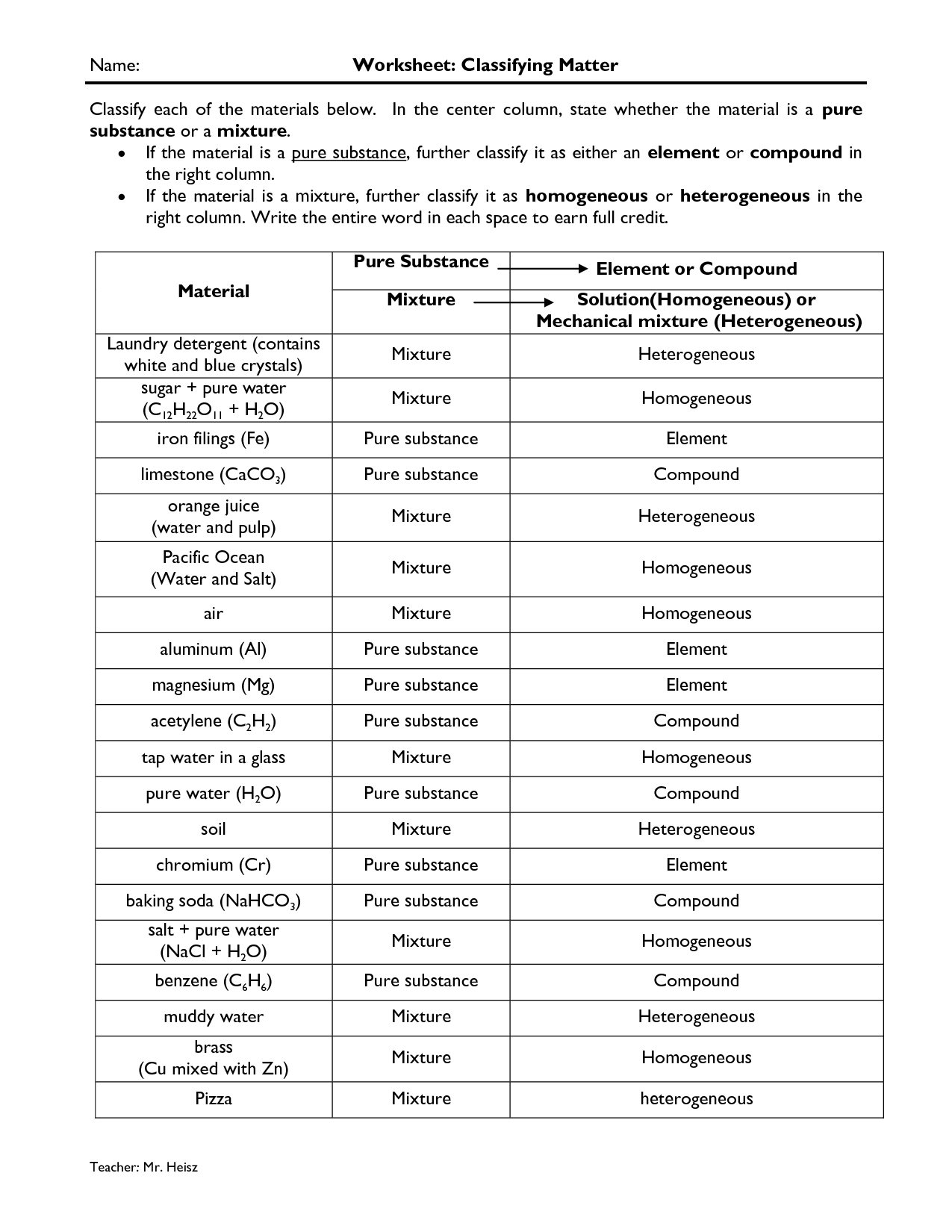
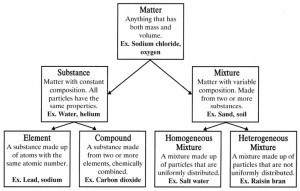
What is the difference between a pure substance and a mixture?
A pure substance consists of only one type of substance with uniform composition and distinct chemical properties, such as elements or compounds. In contrast, a mixture is made up of two or more different substances physically combined, each retaining their individual properties and not chemically bonded, such as solutions, suspensions, or colloids.
What is an element?
An element is a substance that cannot be chemically broken down into simpler substances and is composed of only one type of atom. Elements are the building blocks of all matter in the universe and are organized in the periodic table based on their atomic number and properties.
What is a compound?
A compound is a substance formed by the chemical combination of two or more elements in fixed proportions. These elements are bonded together in specific ratios, creating a distinct and unique chemical structure with its own properties different from the elements that make it up.
Explain the difference between a homogeneous mixture and a heterogeneous mixture.
A homogeneous mixture is a uniform mixture where the components are evenly distributed and not easily distinguished, such as salt dissolved in water. In contrast, a heterogeneous mixture is a non-uniform mixture where the components are unevenly distributed and can be visibly distinguished, such as a salad with different vegetables and dressing.
What is the difference between a solution and a suspension?
A solution is a homogenous mixture where one or more substances (solutes) are dissolved in another substance (solvent), resulting in a clear and transparent liquid. A suspension, on the other hand, is a heterogeneous mixture where fine particles of a solid are dispersed in a liquid, resulting in a cloudy or opaque mixture that eventually settles over time. In summary, solutions are clear and uniform mixtures, while suspensions are cloudy and contain visible particles that do not dissolve.
What is the particle size in a solution?
Particle size in a solution refers to the physical dimensions of particles suspended in a liquid. These particles can vary in size, ranging from a few nanometers to several micrometers. The measurement of particle size is important in various industries such as pharmaceuticals, cosmetics, and food processing as it can impact the stability, appearance, and performance of the solution.
Explain the concept of solubility.
Solubility is the ability of a substance to dissolve in a solvent to form a homogeneous solution. It is determined by the interactions between the solute and solvent molecules. Substances that readily dissolve in a solvent are considered soluble, while those that do not dissolve are considered insoluble. The solubility of a substance can be affected by factors such as temperature, pressure, and the chemical properties of the solute and solvent.
What is distillation and how does it work?
Distillation is a separation process that involves the heating of a liquid mixture to create vapor and then cooling the vapor back to liquid form to separate and purify different components based on their boiling points. As the liquid mixture is heated in a distillation apparatus, components with lower boiling points vaporize first and rise to a condenser where they are cooled and collected, while components with higher boiling points remain in liquid form. This process allows for the separation of substances in the mixture based on their different boiling points, enabling the purification of liquids or isolation of specific components.
What are the physical properties used to classify matter?
Some physical properties used to classify matter include color, shape, size, density, texture, odor, taste, melting point, boiling point, solubility, conductivity, and malleability. These properties help us distinguish between different substances and understand their behavior under various conditions.
Explain the concept of chemical properties.
Chemical properties refer to the inherent characteristics of a substance that describe how it interacts with other substances to undergo a chemical reaction or change in composition. These properties are determined by the substance's chemical composition and structure, and they include traits such as reactivity, flammability, toxicity, and stability. Chemical properties help to identify and distinguish different substances, as well as understand how they will behave in various chemical processes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments