Chemistry Worksheets with Answer Key
Chemistry is a subject that requires thorough understanding and practice to excel in. To help students grasp complex concepts and reinforce their learning, Chemistry worksheets with answer keys can be extremely helpful. By providing a structured format for practicing various topics and offering solutions for immediate feedback, these worksheets aid students in their learning journey. Whether you are a high school student preparing for exams or a teacher looking for supplementary resources, Chemistry worksheets with answer keys can be a valuable tool to enhance your understanding of this fascinating scientific field.
Table of Images 👆
- Chemistry Stoichiometry Worksheet Answer Key
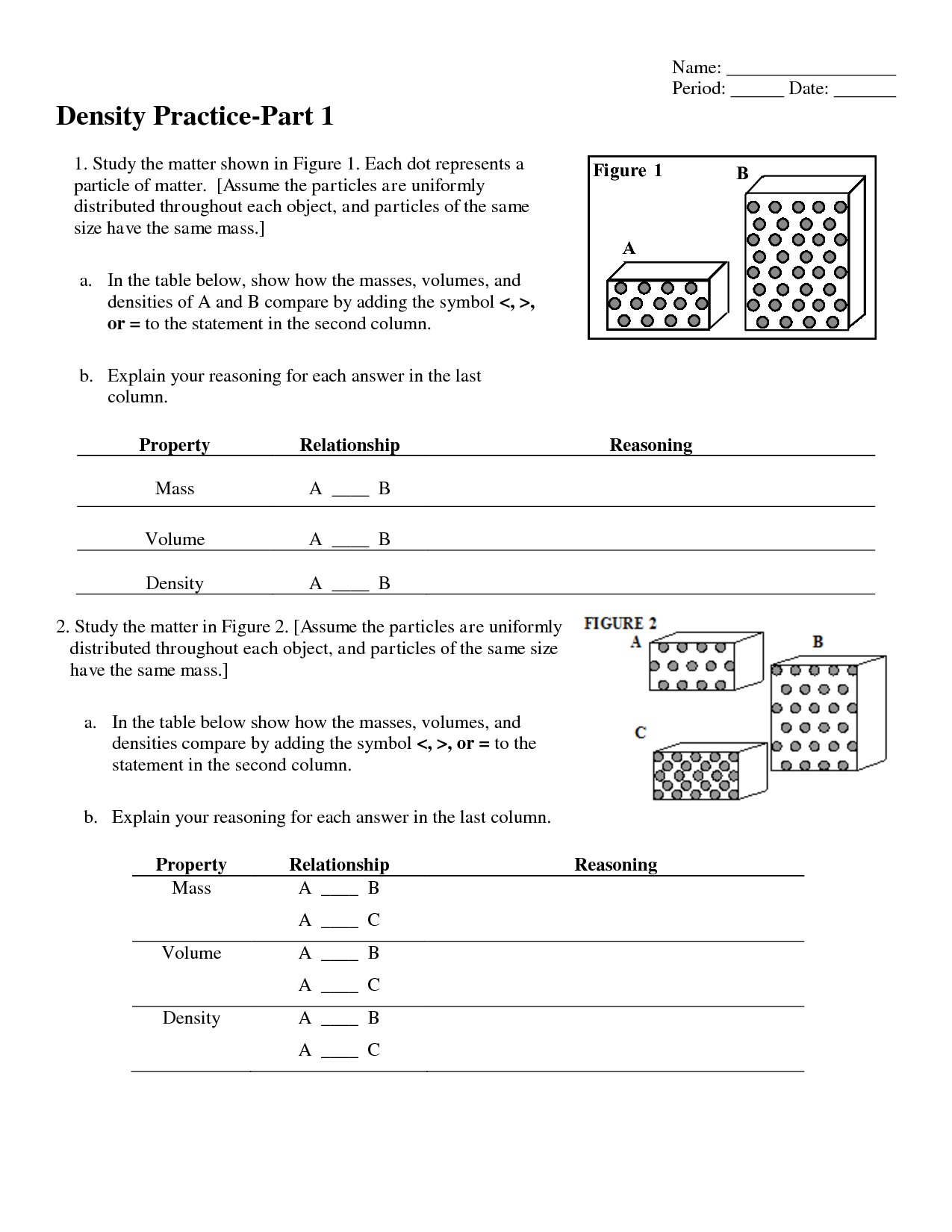
- Chemistry Worksheet Matter 1 Answer Key
- Chemistry Unit 1 Worksheet 3 Answers
- Chemistry Stoichiometry Worksheet Answer Key
- Worksheets Answer Key
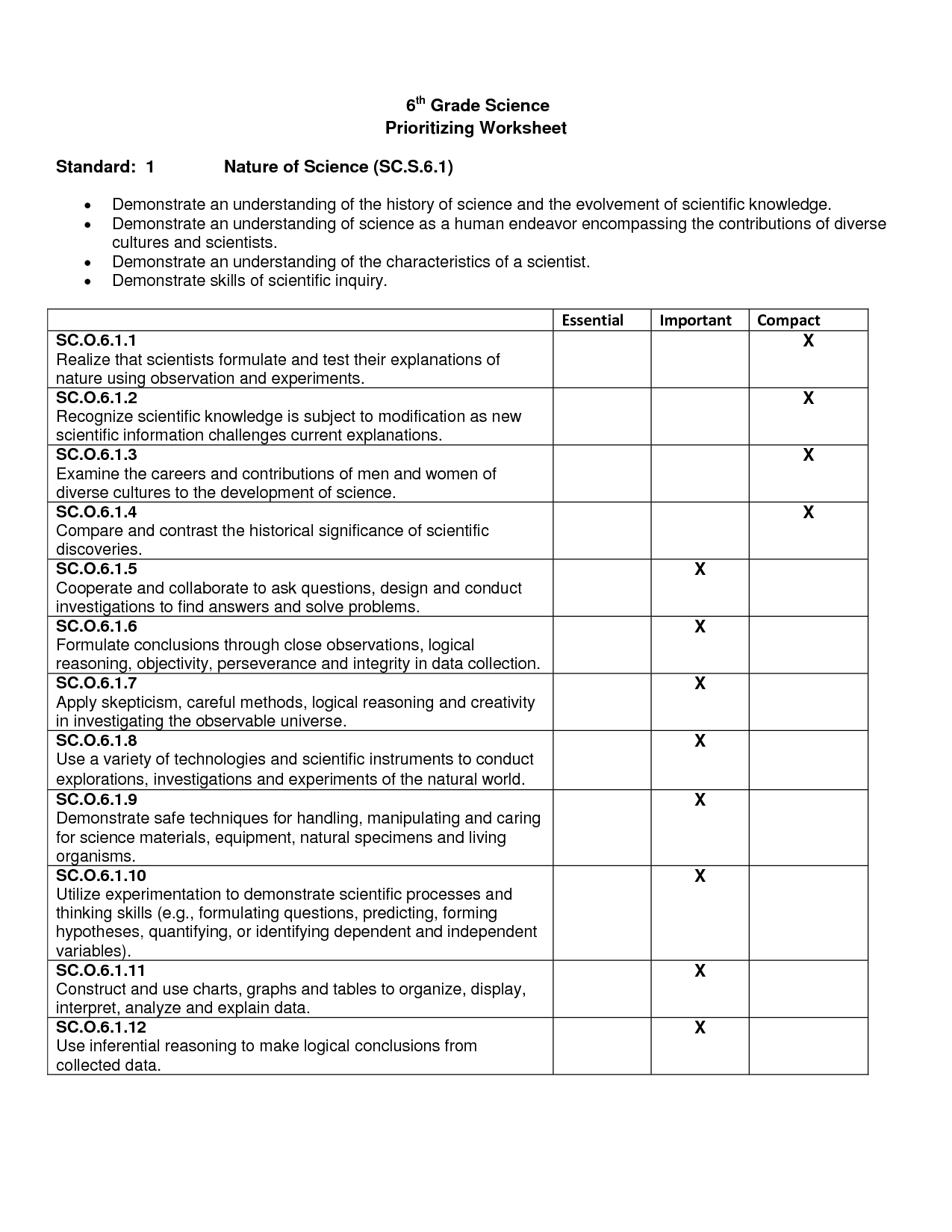
- 6th Grade Science Worksheets with Answer Key
- Naming Ionic Compounds Worksheet Answer Key
- Chemistry Worksheet Matter 1 Answer Key
- Chemistry Unit 5 Worksheet 2 Answer Key
- Periodic Trends Worksheet Answer Key
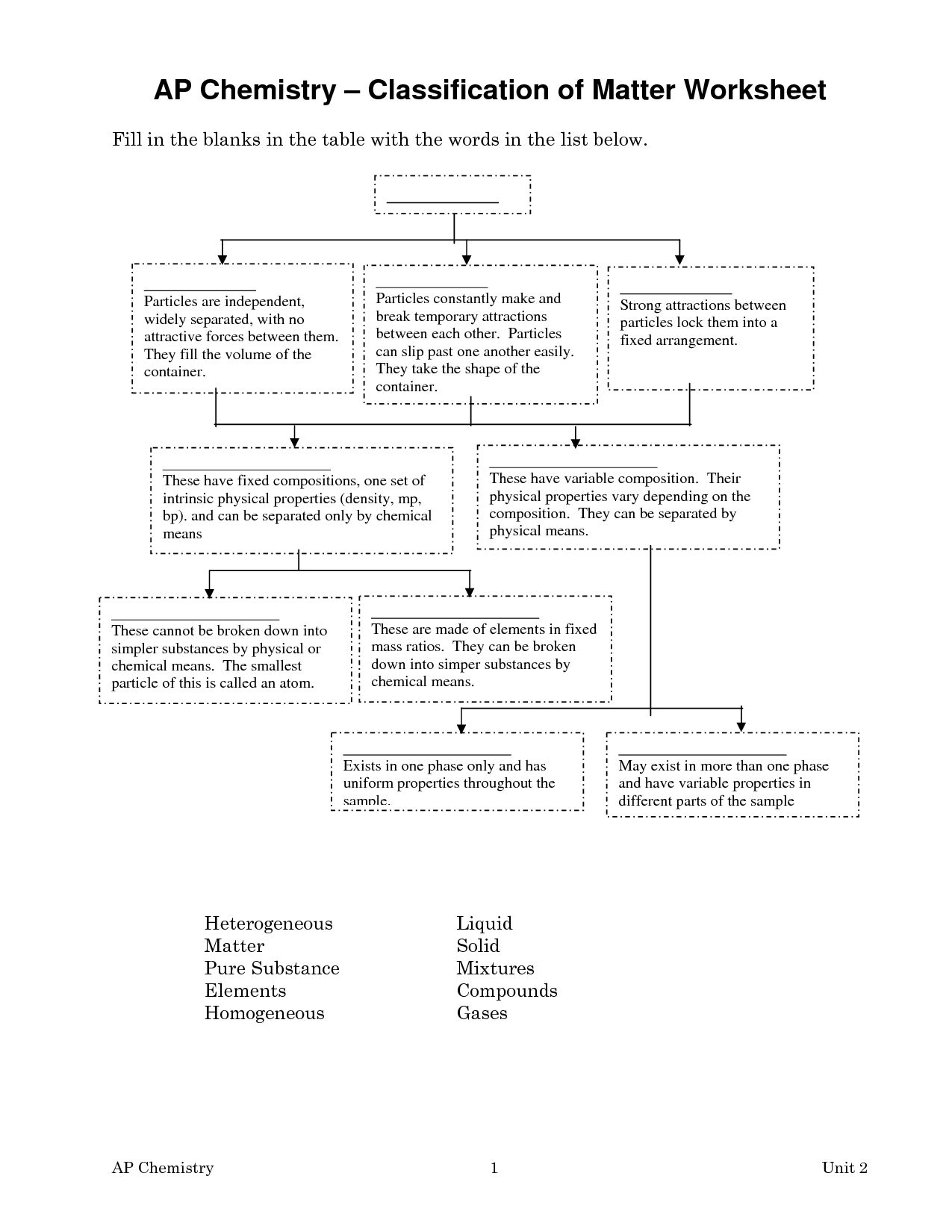
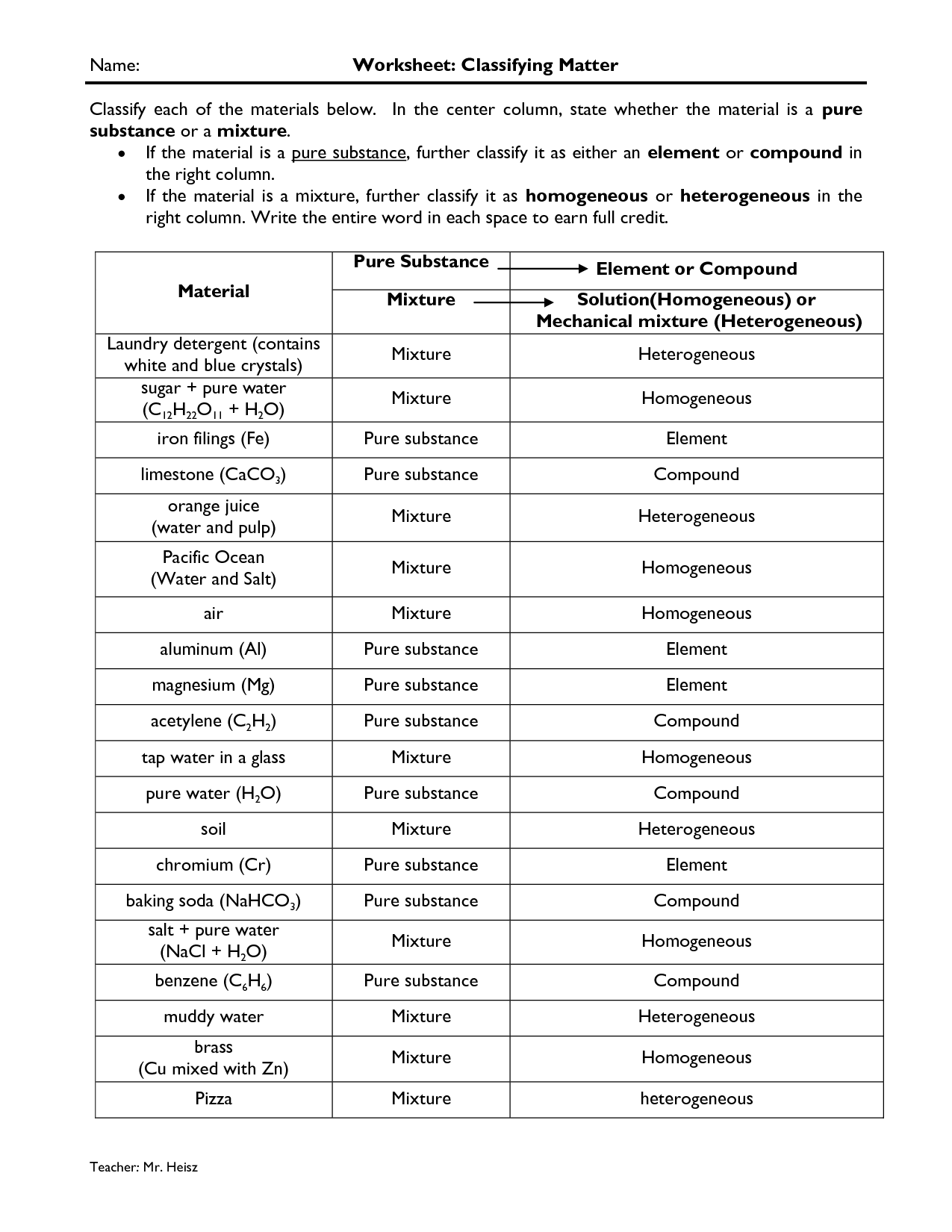
- Classifying Matter Worksheet Answers
- Chemistry Worksheet Matter 1 Answer Key
- Bonding Worksheet Answer Key
- Gas Laws Worksheet Answer Key
- Chemistry Worksheet Matter 1 Answer Key
- Mass to Mole Stoichiometry Worksheet Answer Key
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the molecular formula of water?
The molecular formula of water is H2O, which means it consists of two hydrogen atoms bonded to one oxygen atom.
Define a chemical reaction.
A chemical reaction is a process in which one or more substances, known as reactants, are converted into one or more different substances, known as products, through the breaking and forming of chemical bonds. This transformation involves the rearrangement of atoms, resulting in the formation of new chemical compounds with distinct properties from the original substances.
What is the atomic number of carbon?
The atomic number of carbon is 6.
List three examples of acids.
Hydrochloric acid, sulfuric acid, acetic acid.
Define an element in the context of chemistry.
In the context of chemistry, an element is a pure substance that cannot be broken down into simpler substances by chemical methods. Each element is characterized by its unique chemical and physical properties, such as its atomic number, atomic weight, and electron configuration. Elements are organized on the periodic table based on their atomic structure and properties.
What is the difference between an exothermic and endothermic reaction?
The main difference between an exothermic and endothermic reaction is the direction of heat flow. In an exothermic reaction, heat is released to the surroundings, resulting in a temperature increase. On the other hand, in an endothermic reaction, heat is absorbed from the surroundings, causing a temperature decrease. Exothermic reactions typically feel warm to the touch, while endothermic reactions may feel cool.
Define pH and explain its scale.
pH is a measure of the acidity or basicity of a solution on a scale ranging from 0 to 14. A pH value of 7 indicates neutrality (neither acidic nor basic), while values below 7 indicate acidity and values above 7 indicate basicity. The pH scale is logarithmic, meaning that each whole number change in pH represents a tenfold change in acidity or basicity. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
Name three types of chemical bonds.
The three types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. Ionic bonds form between positively and negatively charged ions, covalent bonds involve the sharing of electron pairs between atoms, and metallic bonds occur between metal atoms where electrons are free to move throughout the structure.
What is the difference between a physical change and a chemical change?
A physical change involves a change in the physical state or appearance of a substance without changing its chemical composition, such as melting ice into water or breaking a glass. In contrast, a chemical change involves the rearrangement of atoms in a substance, resulting in the formation of new substances with different chemical compositions, such as rusting of iron or burning wood.
Define an alloy and provide an example.
An alloy is a mixture of two or more metals, or a metal and a non-metal, that is produced by blending and melting them together to create a new material with enhanced properties compared to its individual components. A common example of an alloy is brass, which is composed of copper and zinc.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments