Chemistry Worksheet Matter 1 Key
Worksheets are tools that provide valuable practice and reinforcement for students. In the field of chemistry, worksheets serve as a means for students to solidify their understanding of key concepts. Whether you are a teacher trying to enhance your lesson plan or a student seeking extra practice, entity worksheets can be a helpful resource.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answer Key
- Chemistry Instructional Fair Worksheet Answers
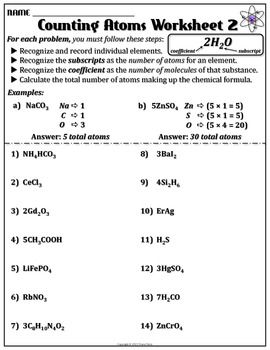
- Atoms and Molecules in Chemical Formulas Worksheet
- Modern Chemistry Chapter 6 Review Answer Key
- Potential Energy Diagrams Chemistry Worksheet
- 17.1 Mechanical Waves Worksheet Answers
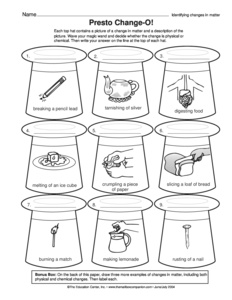
- Matter Physical and Chemical Changes Worksheet
- Chemistry Element Crossword Puzzle
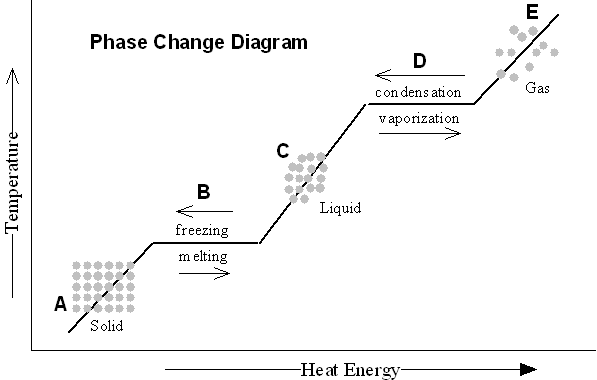
- Blank Phase Change Diagram
- Activities for High School Chemistry Gas Variables POGIL Answer Key
- Activities for High School Chemistry Gas Variables POGIL Answer Key
- Activities for High School Chemistry Gas Variables POGIL Answer Key
- Activities for High School Chemistry Gas Variables POGIL Answer Key
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the scientific definition of matter?
Matter is anything that occupies space and has mass, composed of atoms and molecules that are the building blocks of all substances.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
How do the particles in a solid move?
In a solid, particles vibrate in fixed positions due to strong intermolecular forces holding them together. While they do not move freely like in liquids or gases, the particles in a solid can still oscillate around their fixed positions, resulting in a small amount of movement.
How do the particles in a liquid move?
The particles in a liquid move in a random and constantly changing manner. They have more freedom of movement compared to a solid, allowing them to flow and take the shape of their container. The particles in a liquid are constantly in motion, bouncing off each other and their container, which gives liquids their fluidity.
How do the particles in a gas move?
The particles in a gas move in constant, random motion. They travel in straight lines until they collide with each other or the walls of their container, changing direction with each collision. The speed and direction of their movement is determined by their kinetic energy and the forces acting upon them.
What is the difference between an element and a compound?
An element is a pure substance made up of only one type of atom, such as oxygen or gold, that cannot be broken down into simpler substances by chemical means. On the other hand, a compound is a substance made up of two or more different elements that are chemically bonded together in a fixed ratio, such as water (H2O) or sodium chloride (NaCl). In summary, elements are composed of only one type of atom, while compounds are composed of two or more elements bonded together.
What are the basic building blocks of elements?
Atoms are the basic building blocks of elements. Each element is made up of a unique type of atom, characterized by a specific number of protons in its nucleus. These atoms can combine with each other to form molecules and compounds, creating the vast array of substances found in nature.
How are elements represented on the periodic table?
Elements are represented on the periodic table by their unique chemical symbol, which is typically a one- or two-letter abbreviation. Each element is organized on the table based on its atomic number (number of protons in its nucleus) and its properties, creating a pattern that shows trends and relationships among elements.
What are physical properties of matter?
Physical properties of matter are characteristics that can be observed or measured without changing the composition of the substance. These properties include color, shape, size, texture, density, melting point, boiling point, solubility, conductivity, and magnetism. They help us identify and classify different substances based on their unique physical attributes.
What are chemical properties of matter?
Chemical properties of matter describe how a substance behaves in reactions with other substances, such as its ability to rust, burn, or react with acids. These properties include characteristics like flammability, reactivity, toxicity, and oxidation state, which determine how a substance will interact with other substances and change its composition.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments