Chemistry Worksheet Matter 1 Answer Key
Worksheets are a valuable learning tool for students studying chemistry, providing the perfect platform to practice and reinforce their knowledge. This Chemistry Worksheet Matter 1 Answer Key is an essential resource for educators and students alike, offering a comprehensive collection of answers to assist learners in understanding the concepts covered in the worksheet. With clear explanations and accurate solutions, this answer key serves as a reliable reference for those seeking assistance in mastering the subject of chemistry.
Table of Images 👆
- Chemistry Worksheets with Answer Key
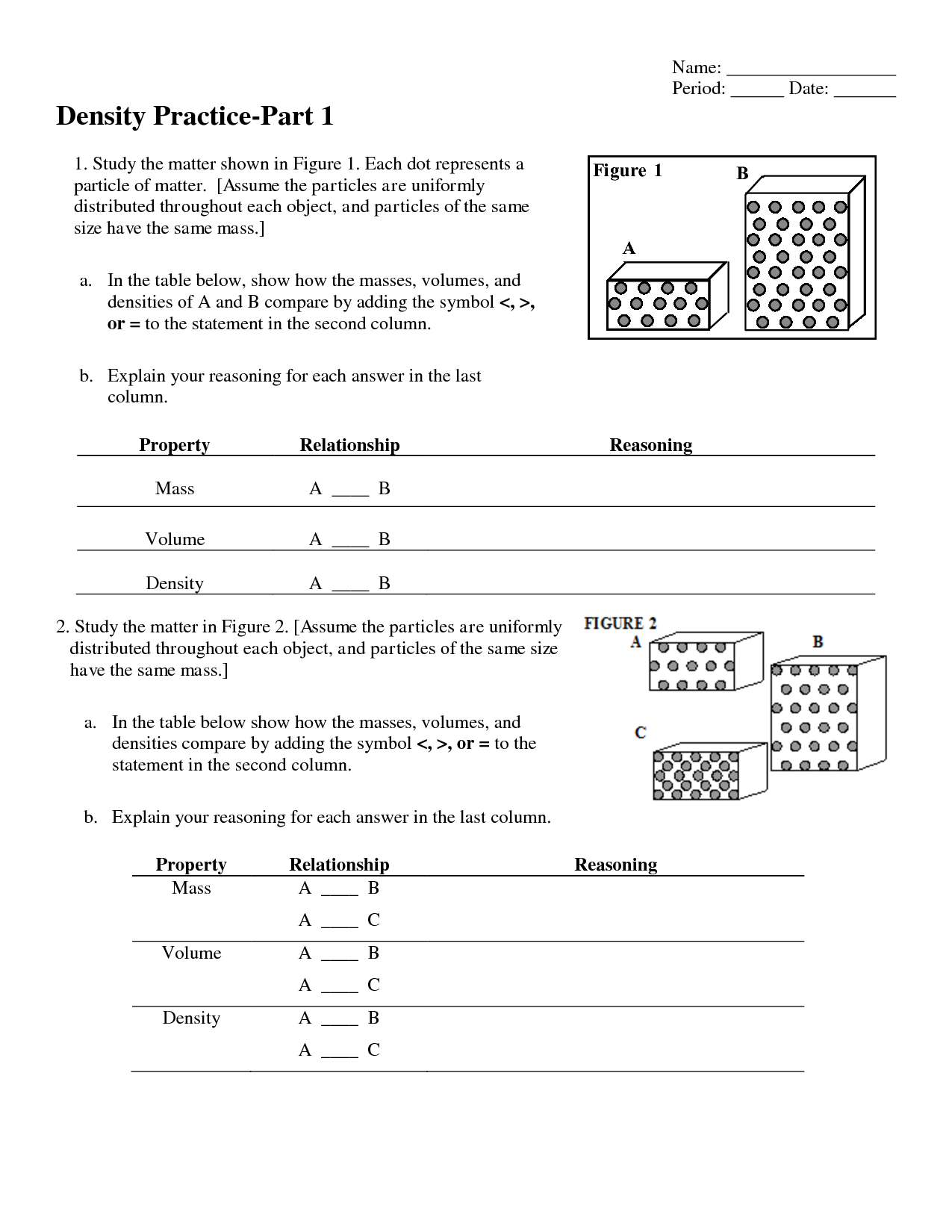
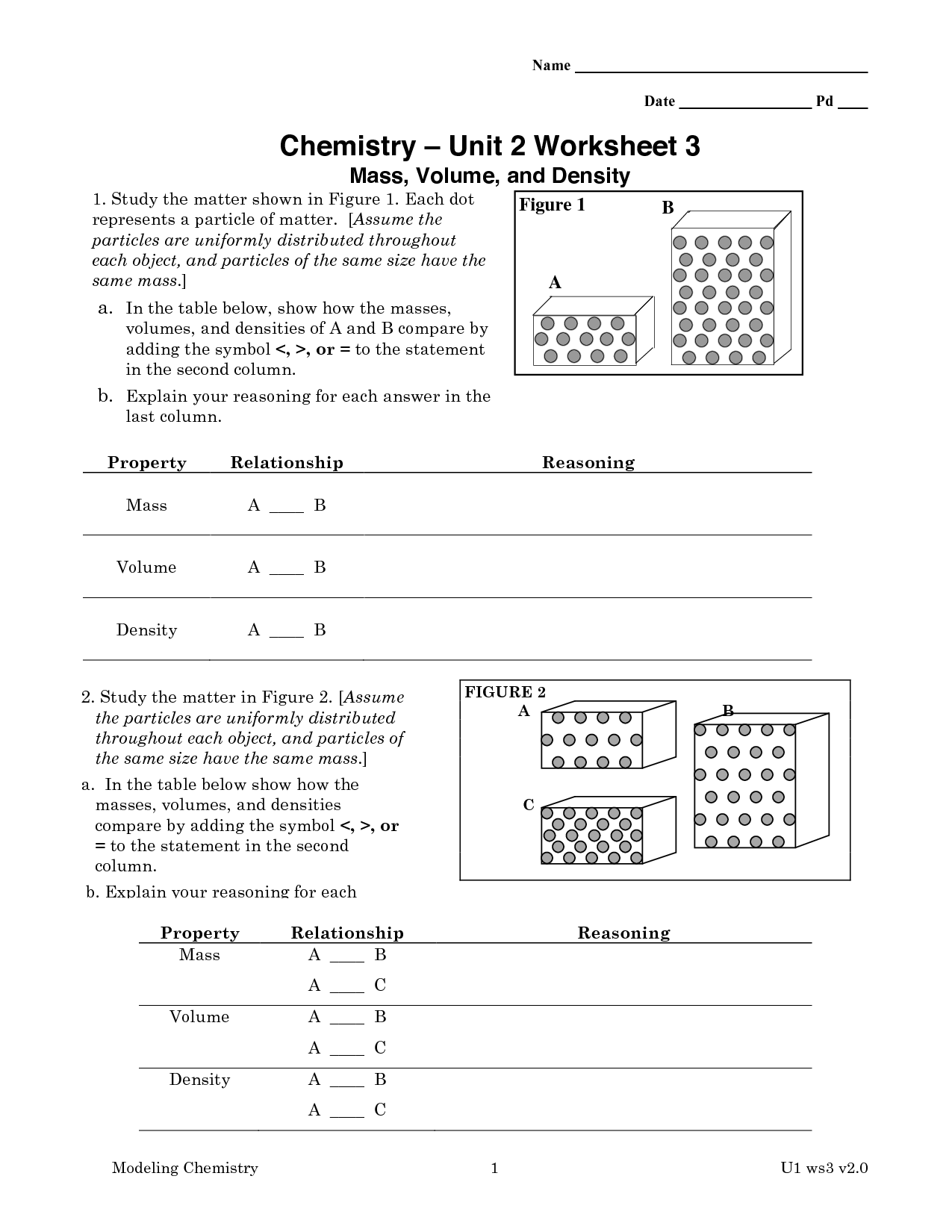
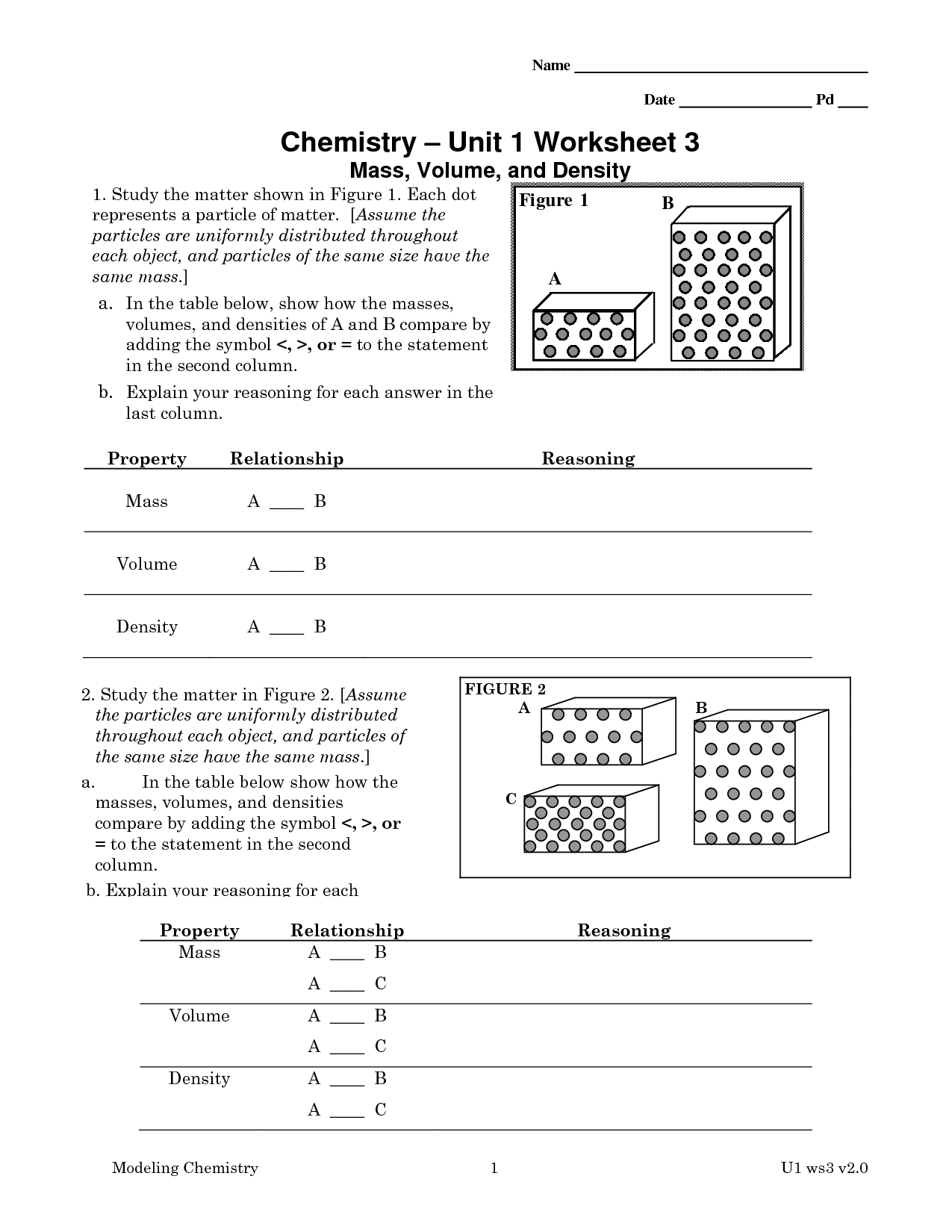
- Chemistry Unit 1 Worksheet 3 Answers
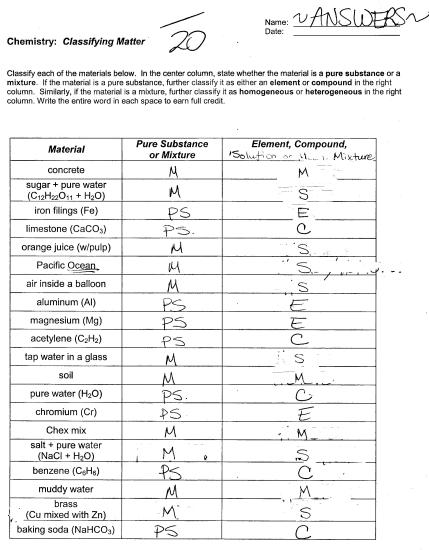
- Classification of Matter Worksheet Answers
- Holt Modern Chemistry Chapter 1 Review Answer Key
- Matter Worksheet Answer Key
- Scientific Notation Worksheet Answers Chemistry
- Chemistry Unit 1 Worksheet 3
- Chemistry Unit 1 Worksheet 3
- Classifying Matter Worksheet Answers
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Stoichiometry Worksheet Answer Key
Chemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the definition of matter?
Matter is anything that has mass and takes up space. It is composed of particles such as atoms and molecules and includes substances like solids, liquids, and gases that make up the physical world around us.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
What is the difference between a physical and chemical change?
A physical change involves a change in the physical state or appearance of a substance without altering its chemical composition, such as melting, freezing, or dissolving. On the other hand, a chemical change involves a transformation at the molecular level that results in the formation of new substances with different chemical properties, such as burning, rusting, or reacting with another substance. Essentially, physical changes are reversible and do not create new substances, whereas chemical changes are irreversible and result in the formation of new substances.
Define an element and give an example.
An element is a substance that cannot be broken down into simpler substances by chemical means. An example of an element is oxygen, which consists of oxygen atoms and is found in the air we breathe.
What is the smallest unit of an element?
The smallest unit of an element is an atom.
What is a compound and give an example.
A compound is a substance formed when two or more elements chemically bond together. One example of a compound is water, which is formed by the chemical bonding of two hydrogen atoms and one oxygen atom (H2O).
How are compounds different from elements?
Compounds are formed by chemical bonding between different elements, resulting in a new substance with unique properties, while elements are pure substances made up of only one type of atom. Compounds can be broken down into their constituent elements through chemical reactions, whereas elements cannot be broken down into simpler substances by chemical means.
Define a mixture and give an example.
A mixture is a combination of two or more substances that are physically combined and can be separated by physical means. An example of a mixture is trail mix, which contains a mixture of nuts, seeds, dried fruits, and sometimes chocolate chips.
How can mixtures be separated?
Mixtures can be separated through various physical methods including filtration, distillation, chromatography, and evaporation. Filtration is used to separate solid particles from liquids, distillation separates substances based on their boiling points, chromatography separates substances based on their different properties, and evaporation is used to separate a dissolved solid from a liquid. Each method exploits the specific physical properties of the components in the mixture to achieve separation.
What is the law of conservation of mass?
The law of conservation of mass states that in a closed system, the total mass remains constant over time. This means that mass cannot be created or destroyed, only transformed from one form to another. In chemical reactions and physical changes, the total mass of the reactants will always be equal to the total mass of the products.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




















Comments