Chemistry Review Worksheets
Chemistry review worksheets are a valuable learning tool for students who want to solidify their understanding of the subject. These worksheets provide practice problems and exercises that cover various topics in chemistry, helping students reinforce key concepts and improve their problem-solving skills. Whether you're a high school student preparing for an exam or a college student wanting to master the intricacies of chemistry, using review worksheets can greatly enhance your learning experience.
Table of Images 👆
- Physical and Chemical Properties Worksheet Answer Key
- Bohr Atomic Model Worksheet
- Combined Gas Law Worksheet Answers
- Periodic Table Worksheet Answers
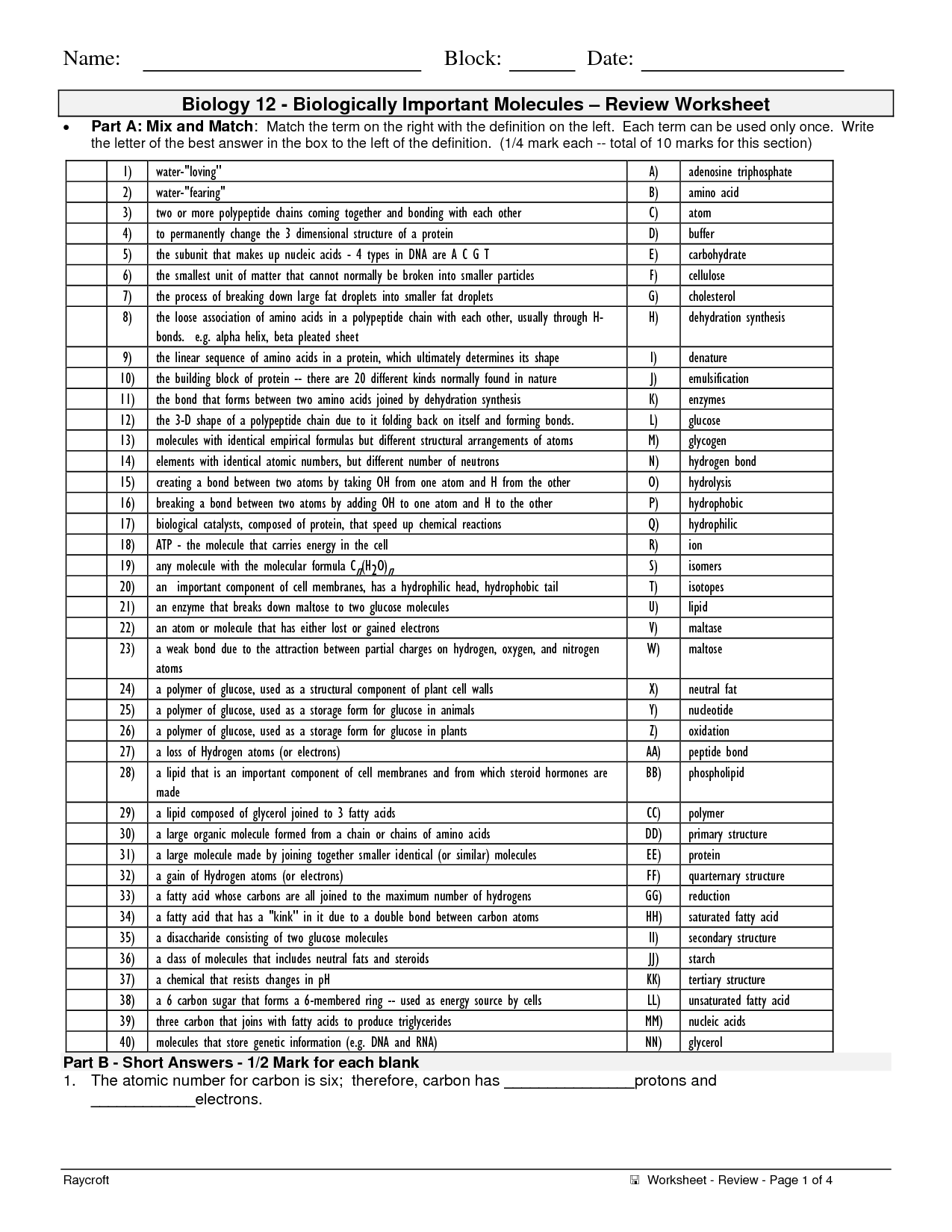
- Organic Compound Worksheet Answers Biology
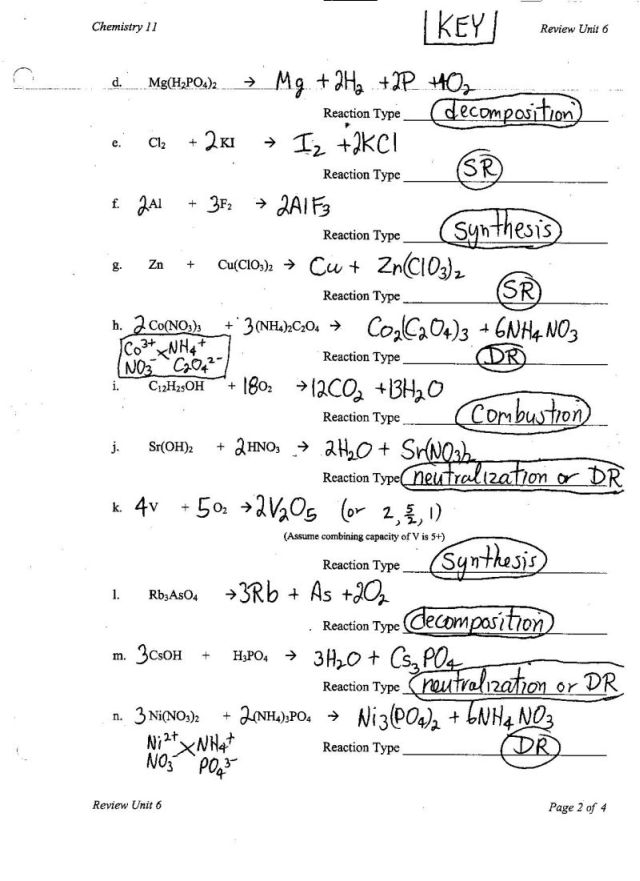
- Types of Chemical Reactions Worksheet Answer Key
- Forensic Science Worksheet Answers
- 3 Year Worksheet Science
- Chemistry Semester 1 Final Exam Study Guide
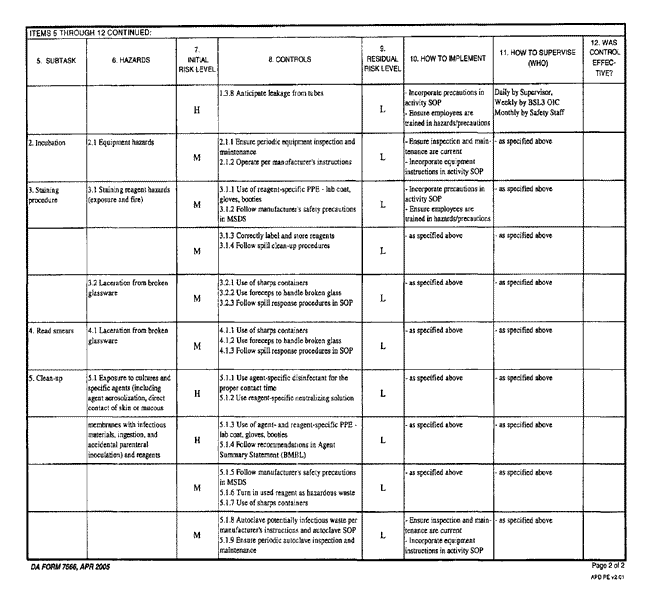
- Army Risk Assessment Worksheet Example
- Mass and Atomic Number Worksheet
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the atomic number?
The atomic number is the number of protons found in the nucleus of an atom, which determines the element's identity on the periodic table.
What is an isotope?
An isotope is an atom of an element that has the same number of protons in its nucleus as other atoms of the same element but has a different number of neutrons. This results in isotopes of an element having different atomic weights.
What is the periodic table?
The periodic table is a tabular arrangement of chemical elements organized by their atomic number, electronic configuration, and recurring chemical properties. It provides a systematic way to categorize elements based on their similarities and helps scientists predict the behavior and properties of various elements.
What is an ion?
An ion is an atom or molecule that has a net electric charge due to the loss or gain of one or more electrons. Ions can be positively charged (cations) if they have lost electrons, or negatively charged (anions) if they have gained electrons.
What is a chemical reaction?
A chemical reaction is a process in which one or more substances (reactants) are converted into one or more new substances (products) by breaking and forming chemical bonds. This process involves the rearrangement of atoms and the transfer of energy, resulting in the formation of different substances with new properties.
What is the difference between an exothermic and endothermic reaction?
An exothermic reaction releases energy in the form of heat, light, or sound, while an endothermic reaction absorbs energy from its surroundings. In exothermic reactions, the products have lower energy than the reactants, resulting in a net release of energy. On the other hand, in endothermic reactions, the products have higher energy than the reactants, requiring energy input to proceed.
What are the three states of matter?
The three states of matter are solid, liquid, and gas. Solid matter has a definite shape and volume, liquid matter has a definite volume but takes the shape of its container, and gas matter has neither a definite shape nor a definite volume, filling the entire space available to it.
What is the law of conservation of mass?
The law of conservation of mass states that in a closed system, the total mass remains constant over time, regardless of any physical or chemical changes that occur within the system. This means that mass is neither created nor destroyed in a chemical reaction, but rather it is rearranged to form new substances.
What are the properties of acids and bases?
Acids are substances that donate protons in a chemical reaction, have a sour taste, turn blue litmus paper red, and have a pH less than 7. Bases are substances that accept protons in a chemical reaction, have a bitter taste, feel slippery, turn red litmus paper blue, and have a pH greater than 7. Both acids and bases are corrosive in high concentrations and can neutralize each other to form salts and water.
What is the difference between a compound and a mixture?
A compound is a pure substance composed of two or more different types of atoms that are chemically bonded together in a fixed ratio, while a mixture is a combination of two or more substances that are physically mixed together but not chemically bonded. In a compound, the constituent elements are chemically combined in a fixed proportion and can only be separated by a chemical reaction, whereas in a mixture, the components can be easily separated through physical means like filtration, evaporation, or distillation.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments