Chemistry Mole Worksheet
Are you struggling to grasp the concept of moles in chemistry? If so, fret not! This blog post is here to help you understand and conquer this fundamental concept. With our carefully crafted chemistry mole worksheet, you can practice solving mole-related problems and enhance your understanding of this essential topic. Designed for students who are just beginning their journey into chemistry, this worksheet is the perfect tool to clarify any doubts you may have and strengthen your grasp on this subject.
Table of Images 👆
- Mole Avogadro Number Worksheets and Answers
- Mole Conversion Worksheet
- Worksheet Mole Problems Answers
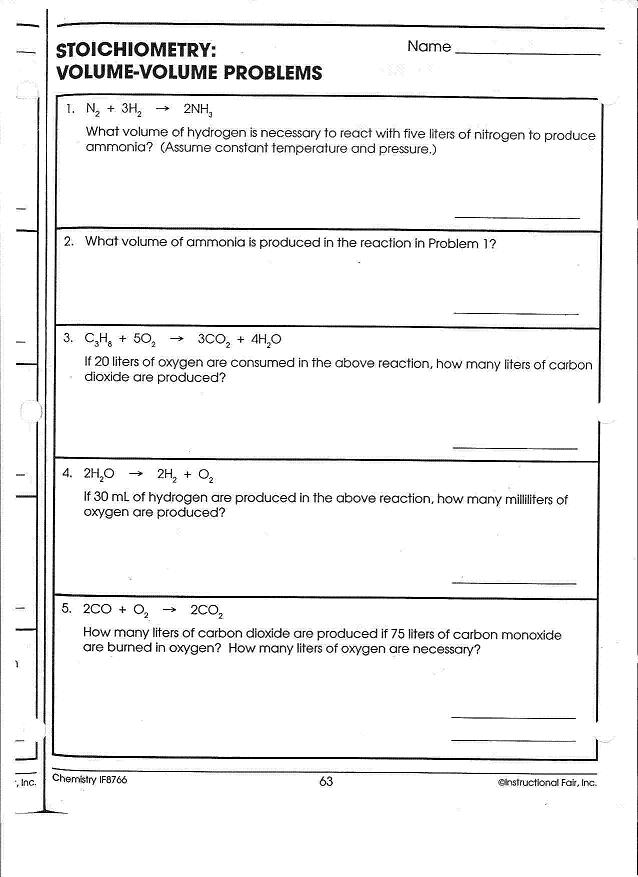
- Chemistry Stoichiometry Worksheet Answers
- Gram Formula Mass Worksheet Answers
- Chemistry Mole Calculation Worksheet
- Chemistry the Mole and Volume Worksheet
- Balancing Chemical Equations Worksheet
- Mass to Mole Stoichiometry Worksheet Answer Key
- Mole Ratio Worksheet Answer Key
- Mole Conversion Problems Worksheet Answers
- Mole Ratio Worksheet Answer Key
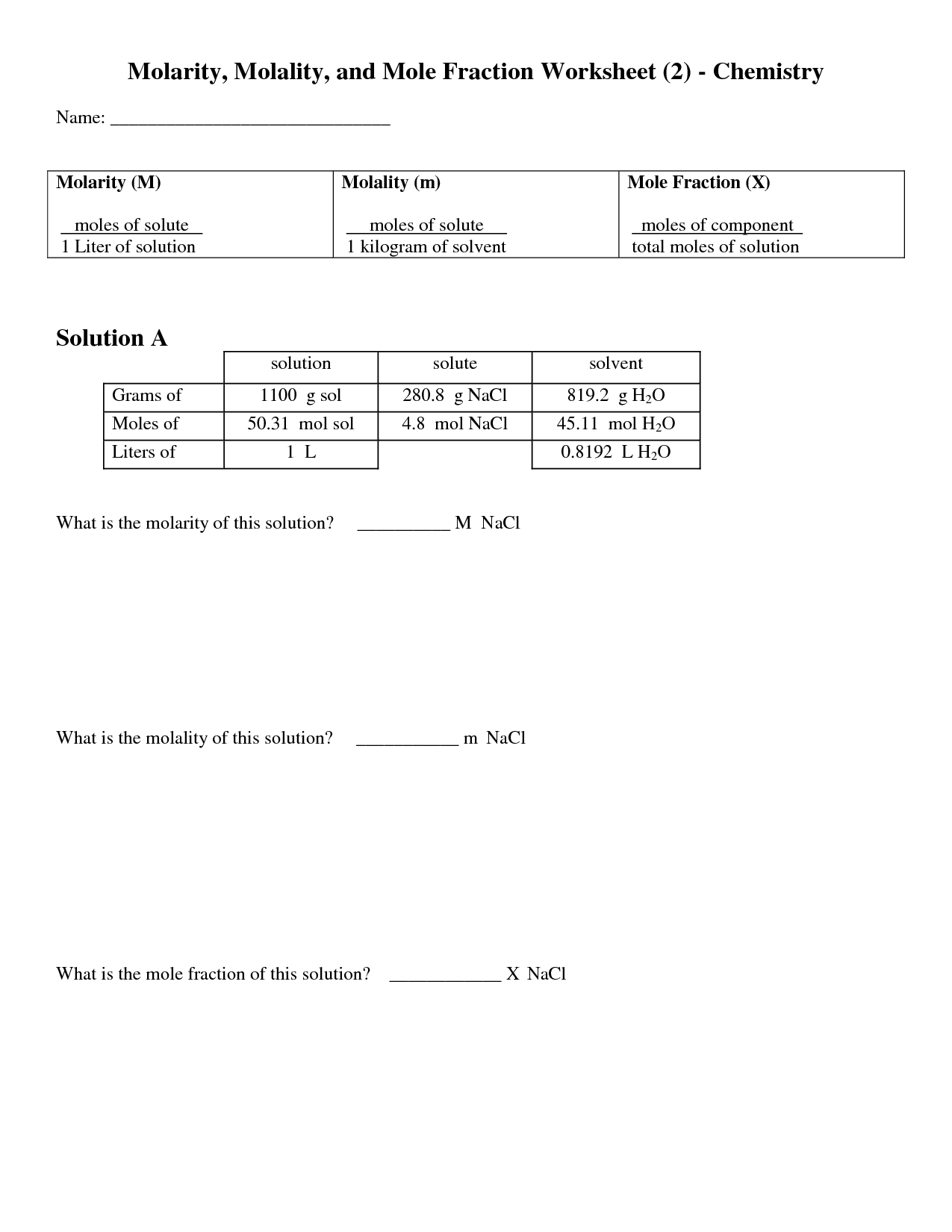
- Molarity Molality Mole Fraction Worksheet
- Mole Stoichiometry Worksheet Answers
- Mole Conversion Worksheet Answers
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is a mole in chemistry?
A mole in chemistry is a unit used to express the amount of a substance. It is defined as the amount of a substance that contains the same number of particles as there are atoms in 12 grams of carbon-12, approximately 6.022 x 10^23 particles. This number is known as Avogadro's number and is used to relate the mass of a substance to the number of atoms or molecules it contains.
What is Avogadro's number?
Avogadro's number is a constant representing the number of constituent particles (usually atoms or molecules) in one mole of a substance, which is approximately 6.022 x 10^23. This number is crucial in chemistry for converting between the mass of a substance and the number of atoms or molecules it contains.
How is the molar mass of a substance calculated?
The molar mass of a substance is calculated by adding up the atomic masses of all the atoms in its chemical formula. This is done by looking at the periodic table to find the atomic mass of each element and then multiplying it by the number of atoms of that element in the compound. These individual atomic masses are then added together to get the molar mass in grams per mole.
What is the relationship between moles and grams for a given substance?
The relationship between moles and grams for a given substance depends on the substance's molar mass. Molar mass is the mass of one mole of a substance and is expressed in grams per mole. To convert between moles and grams, you can use the molar mass of the substance as a conversion factor. By multiplying the number of moles by the molar mass, you can determine the mass of the substance in grams, or by dividing the mass in grams by the molar mass, you can calculate the number of moles. This relationship allows for easy conversion between moles and grams for a given substance.
How can you convert between moles and particles?
To convert between moles and particles, you can use Avogadro's number, which is 6.022 x 10^23 particles per mole. To convert from moles to particles, multiply the number of moles by Avogadro's number. To convert from particles to moles, divide the number of particles by Avogadro's number. This allows you to easily switch between the two units when working with quantities of atoms, molecules, or ions.
What is the difference between empirical formula and molecular formula?
The empirical formula represents the simplest whole-number ratio of elements in a compound, while the molecular formula gives the actual number of atoms of each element present in a molecule of the compound. This means that the empirical formula may not always match the molecular formula, as the molecular formula gives the exact composition of the molecule whereas the empirical formula simplifies it to the smallest whole numbers.
How can you determine the percent composition of a compound?
To determine the percent composition of a compound, you need to find the mass of each element in the compound and then divide it by the total molar mass of the compound. Multiply the result by 100 to get the percentage. This calculation will give you the percentage of each element present in the compound by mass.
What is stoichiometry and how is it related to moles?
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. It involves the calculation of the amounts of reactants and products based on balanced chemical equations. The concept of moles is crucial in stoichiometry because it is used to convert between the mass of a substance and the number of particles (atoms, molecules, ions) it contains. By using the mole ratio from a balanced chemical equation, one can determine the amounts of substances involved in a reaction and predict the yields of products.
What is the purpose of using balanced chemical equations in mole calculations?
The purpose of using balanced chemical equations in mole calculations is to establish the proper stoichiometric ratios between reactants and products. Balancing the equation ensures that the amounts of each substance are accurately represented, allowing for precise calculations of moles and enabling predictions about the quantities of substances involved in a reaction. This helps ensure that reactions are carried out efficiently and with the correct proportions of reactants.
How can you use the mole concept to determine the limiting reactant in a chemical reaction?
To determine the limiting reactant in a chemical reaction using the mole concept, you first need to convert the amount of each reactant given (in grams, moles, or any other unit) to moles. Then, using the balanced chemical equation and stoichiometry, you can calculate the theoretical amount of product that each reactant can produce. The reactant which produces the least amount of product is the limiting reactant. This is because once all of the limiting reactant is consumed, the reaction cannot continue further, even if there is excess of the other reactant remaining.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments