Chemistry Gas Laws Worksheet
If you're in need of a comprehensive and informative resource to reinforce your understanding of chemistry gas laws, then this Chemistry Gas Laws Worksheet is just what you need. Designed with students in mind, this worksheet delves into the principles and calculations involved in gas laws, allowing you to practice and solidify your knowledge on this subject.
Table of Images 👆
- Ideal Gas Law Worksheet Answer Key
- Ideal Gas Law Worksheet Answers
- Charles Law Worksheet Answers
- Chapter 11 Worksheet 1 Chemistry
- Combined Gas Law Worksheet Answers
- Ideal Gas Law Worksheet Answers
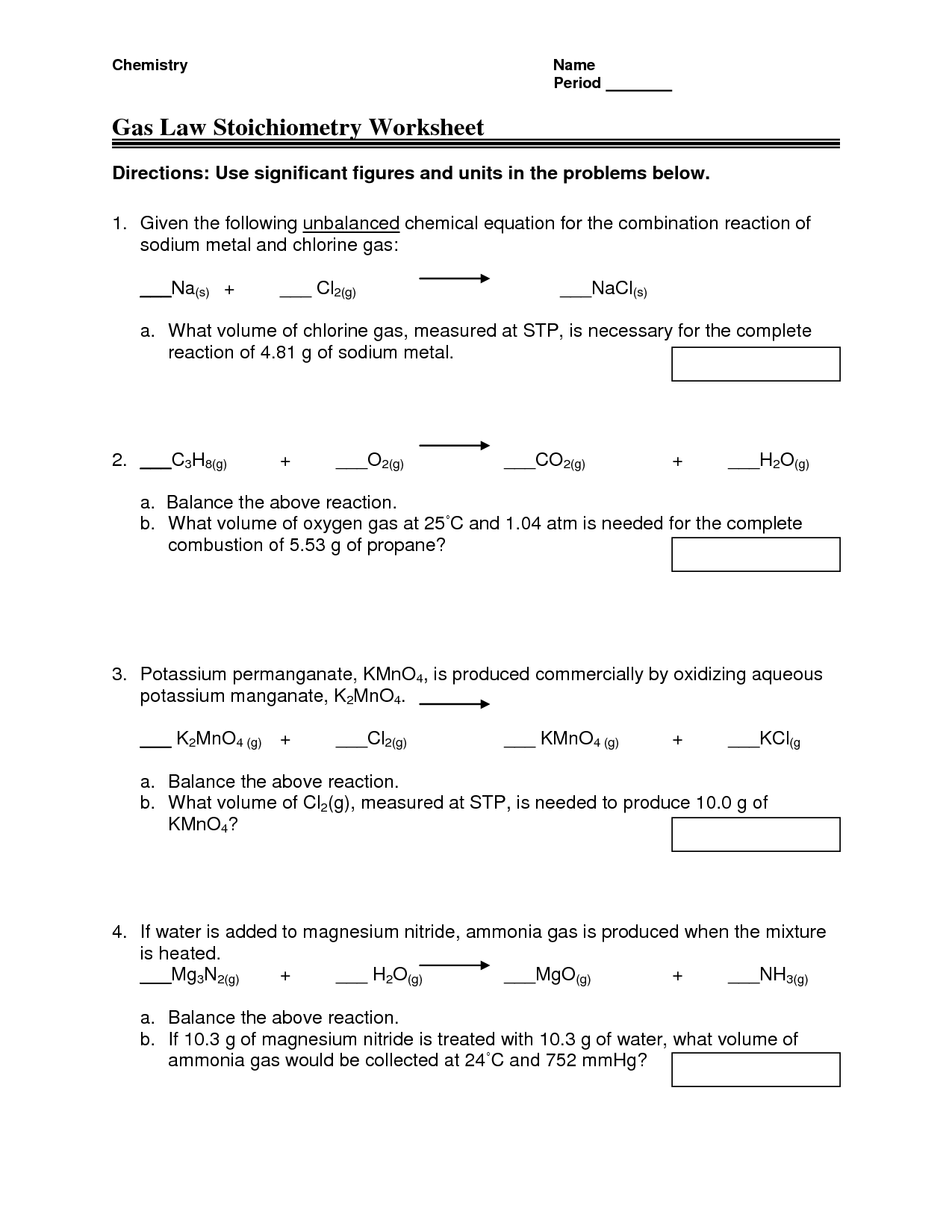
- Gas Law Stoichiometry Worksheet
- Gases and Thermochemistry Worksheets
- Combined Gas Law Worksheet
- Ideal Gas Law Worksheet Answer Key
- Gas Laws Worksheet
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the ideal gas law?
The ideal gas law is a fundamental equation in thermodynamics that relates the pressure, volume, and temperature of a gas. It is described by the formula PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
What are the four variables in the ideal gas law equation?
The four variables in the ideal gas law equation are pressure (P), volume (V), temperature (T), and the number of moles of gas (n). The equation, PV = nRT, describes the relationship between these variables for an ideal gas under given conditions.
How does Charles's law relate temperature and volume?
Charles's law states that the volume of a gas is directly proportional to its temperature, assuming pressure and amount of gas remain constant. This means that as the temperature of a gas increases, its volume also increases, and as the temperature decreases, the volume decreases proportionally. This relationship helps explain why hot air balloons rise - as the air inside the balloon is heated, its volume expands, making the balloon less dense than the surrounding air, causing it to float.
What is Avogadro's law?
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. In other words, the volume of a gas is directly proportional to the number of moles of gas present. This law is important in understanding the behavior of gases and is used in various calculations in the field of chemistry.
How is Boyle's law different from Charles's law?
Boyle's law describes the relationship between the pressure and volume of a gas at constant temperature, stating that pressure and volume are inversely proportional. Charles's law, on the other hand, relates the volume and temperature of a gas at constant pressure, stating that volume is directly proportional to temperature. In summary, Boyle's law focuses on the relationship between pressure and volume, while Charles's law focuses on the relationship between volume and temperature of a gas under different conditions.
What is the relationship between pressure and volume in Boyle's law?
Boyle's law states that the pressure of a gas is inversely proportional to its volume, assuming the temperature and amount of gas remain constant. This means that as the volume of a gas decreases, the pressure of the gas increases, and vice versa. Mathematically, this relationship is expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
How does the combined gas law combine Boyle's, Charles's, and Avogadro's laws?
The combined gas law combines Boyle's, Charles's, and Avogadro's laws by showing the relationship between pressure, volume, temperature, and the number of moles of a gas. It states that when the temperature, pressure, and volume of a gas change, the product of the pressure and volume divided by the temperature is constant, as long as the number of moles of gas remains constant. This equation helps us understand how these three gas laws work together to describe the behavior of gases under different conditions.
What is the formula for calculating the density of a gas?
The formula for calculating the density of a gas is density (?) = (mass (m) / volume (V)) * molar mass (M), where the mass is typically measured in grams, volume in liters, and molar mass in g/mol.
How does Gay-Lussac's law relate pressure and temperature?
Gay-Lussac's law states that the pressure of a gas is directly proportional to its temperature when the volume remains constant. This means that as the temperature of a gas increases, its pressure also increases in a linear relationship. Conversely, if the temperature decreases, the pressure of the gas will also decrease. This principle is key to understanding the behavior of gases when subjected to changes in temperature and pressure.
How can the ideal gas law be used to calculate the number of moles or the molar mass of a gas?
The ideal gas law, PV = nRT, can be rearranged to calculate the number of moles (n) by dividing the given pressure (P) times volume (V) by the gas constant (R) times temperature (T). To find the molar mass of a gas, one can rearrange the ideal gas law to solve for molar mass (M) by dividing the product of the gas constant (R) times temperature (T) by the pressure (P) times volume (V) times the number of moles (n). This allows for calculating the molar mass of a gas based on known pressure, volume, temperature, and the number of moles.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments