Chemical Reactions Worksheet with Answers
Chemical reactions are an essential aspect of chemistry education, allowing students to understand how substances react and change. For educators looking to supplement their lessons with practical exercises, a chemical reactions worksheet with answers can be an invaluable resource. These worksheets provide a structured format for students to practice identifying reactants, products, and balancing equations. By offering a comprehensive overview of this fundamental topic, these worksheets cater to high school and college students seeking to solidify their understanding of chemical reactions.
Table of Images 👆
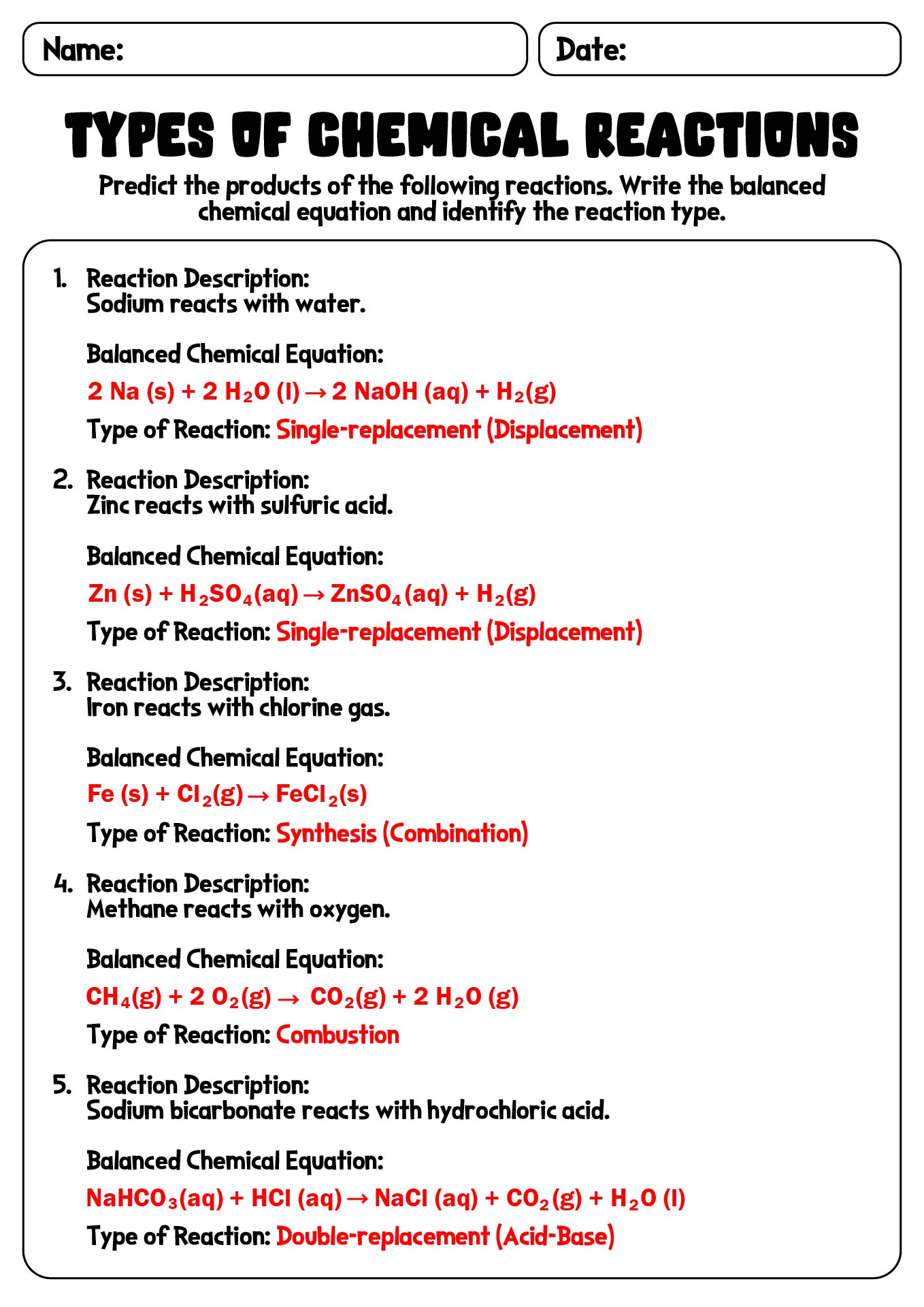
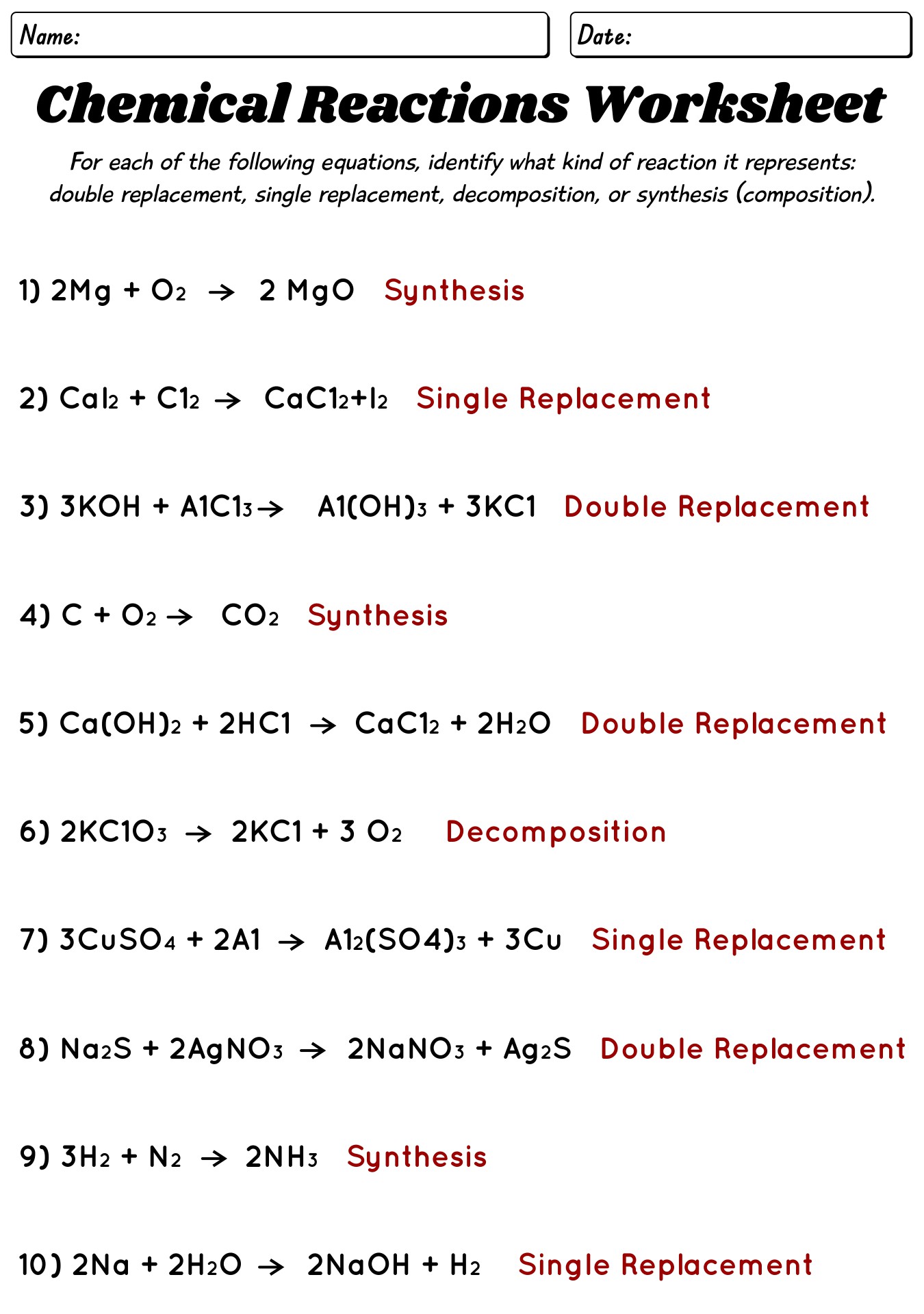
- Types Chemical Reactions Worksheets Answers
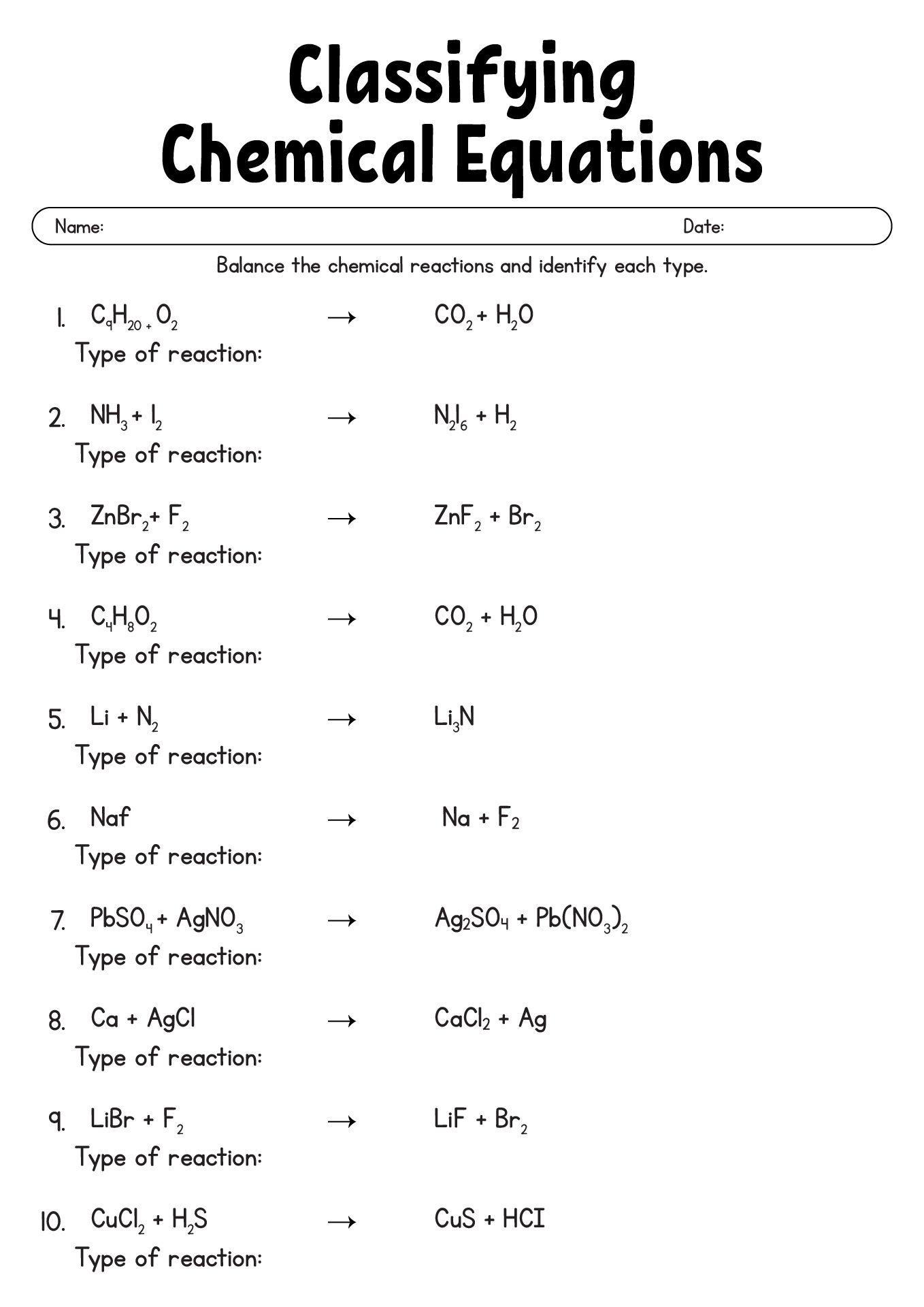
- Classifying Chemical Reactions Worksheet Answers
- Chemical Reaction Types Worksheet
- Types of Chemical Reactions Worksheet Answer Key
- Chemistry Worksheet Answer Keys
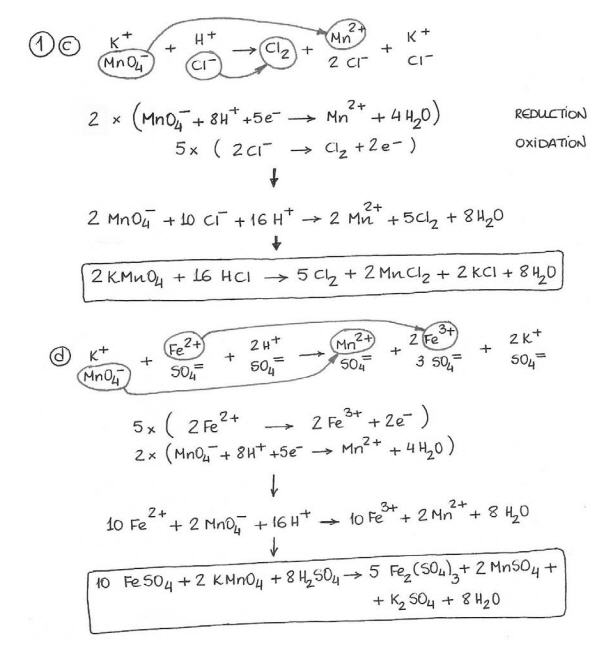
- Practice Balancing Redox Reaction Equations
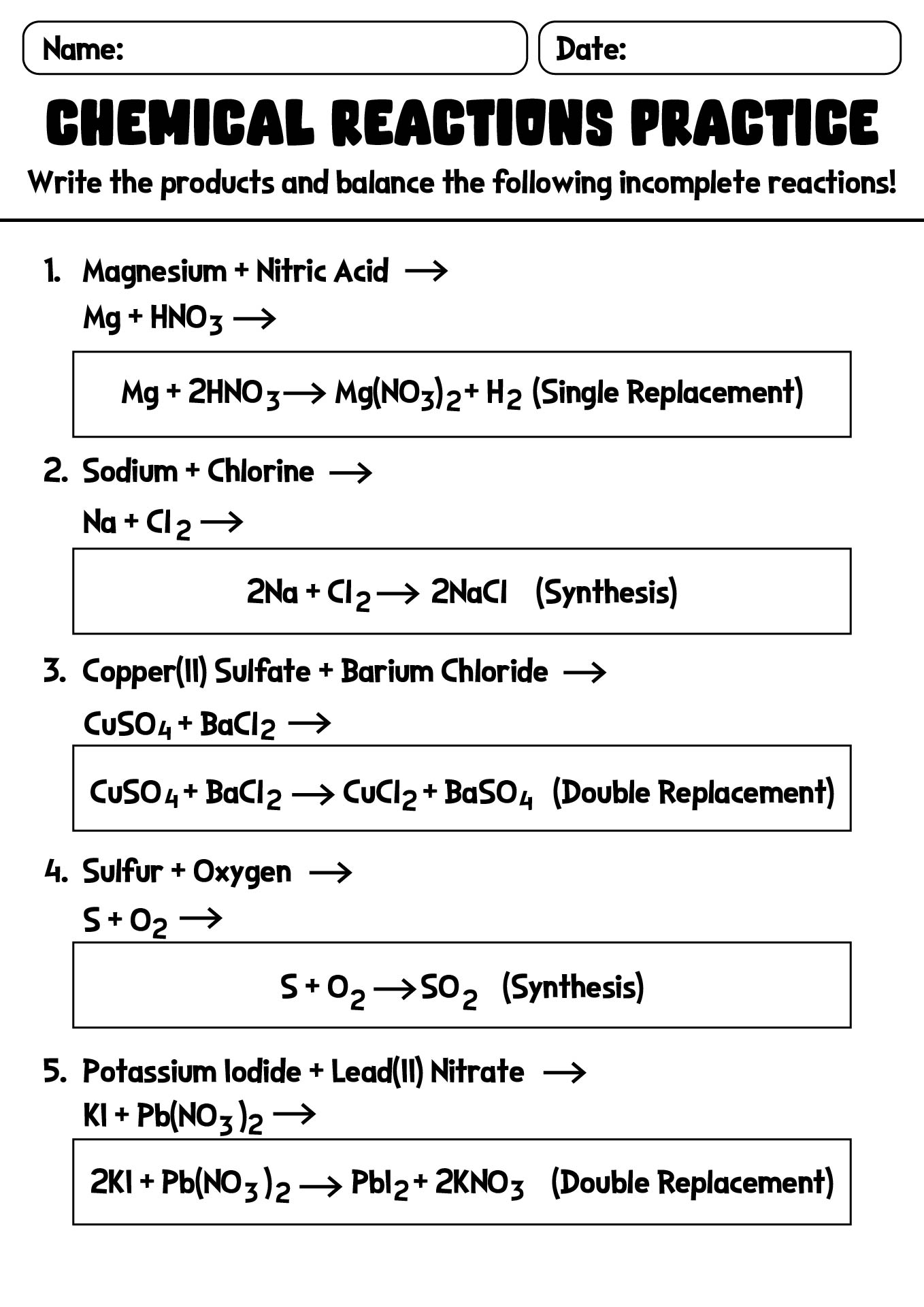
- Chemical Reactions Practice Worksheet with Solutions
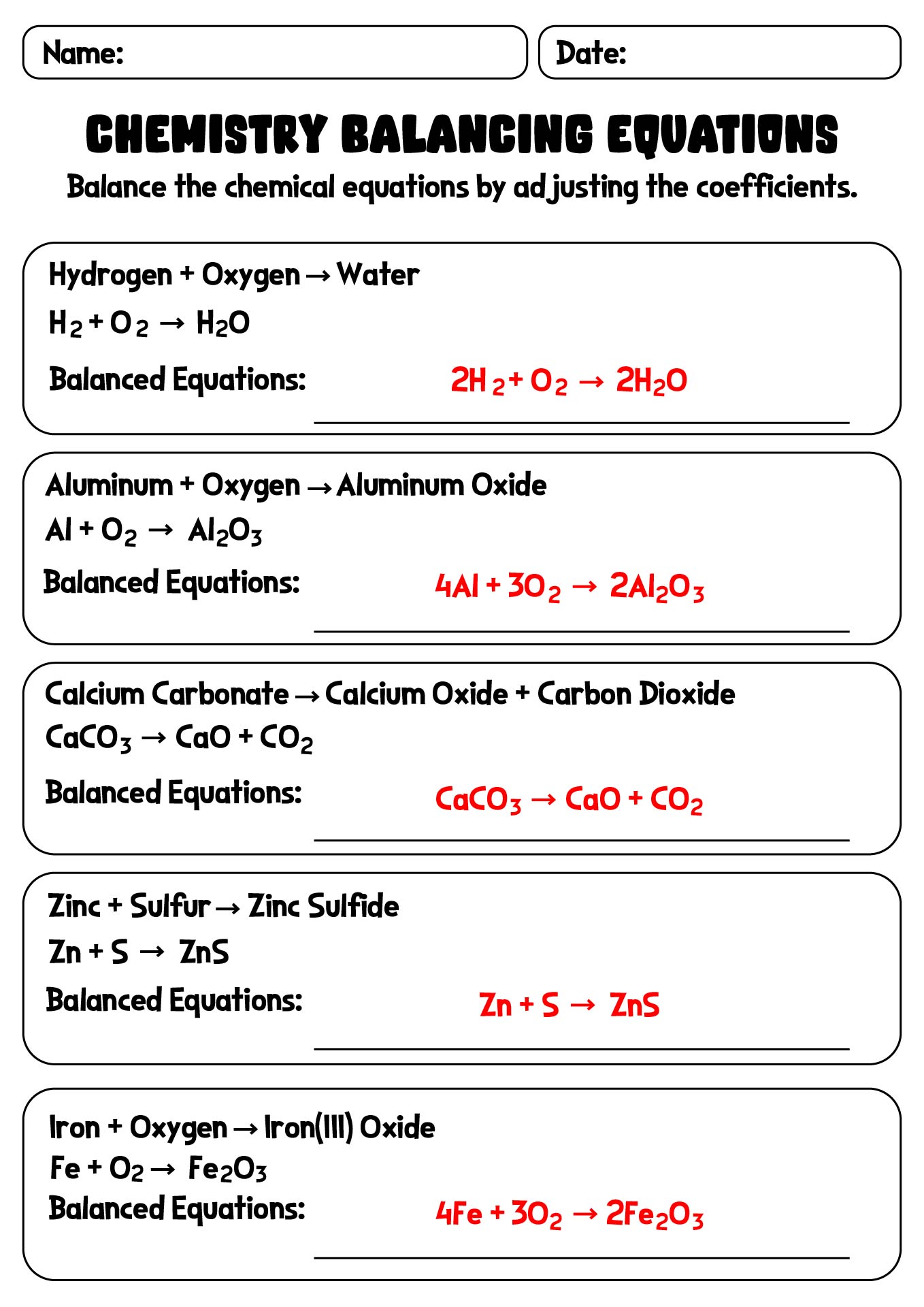
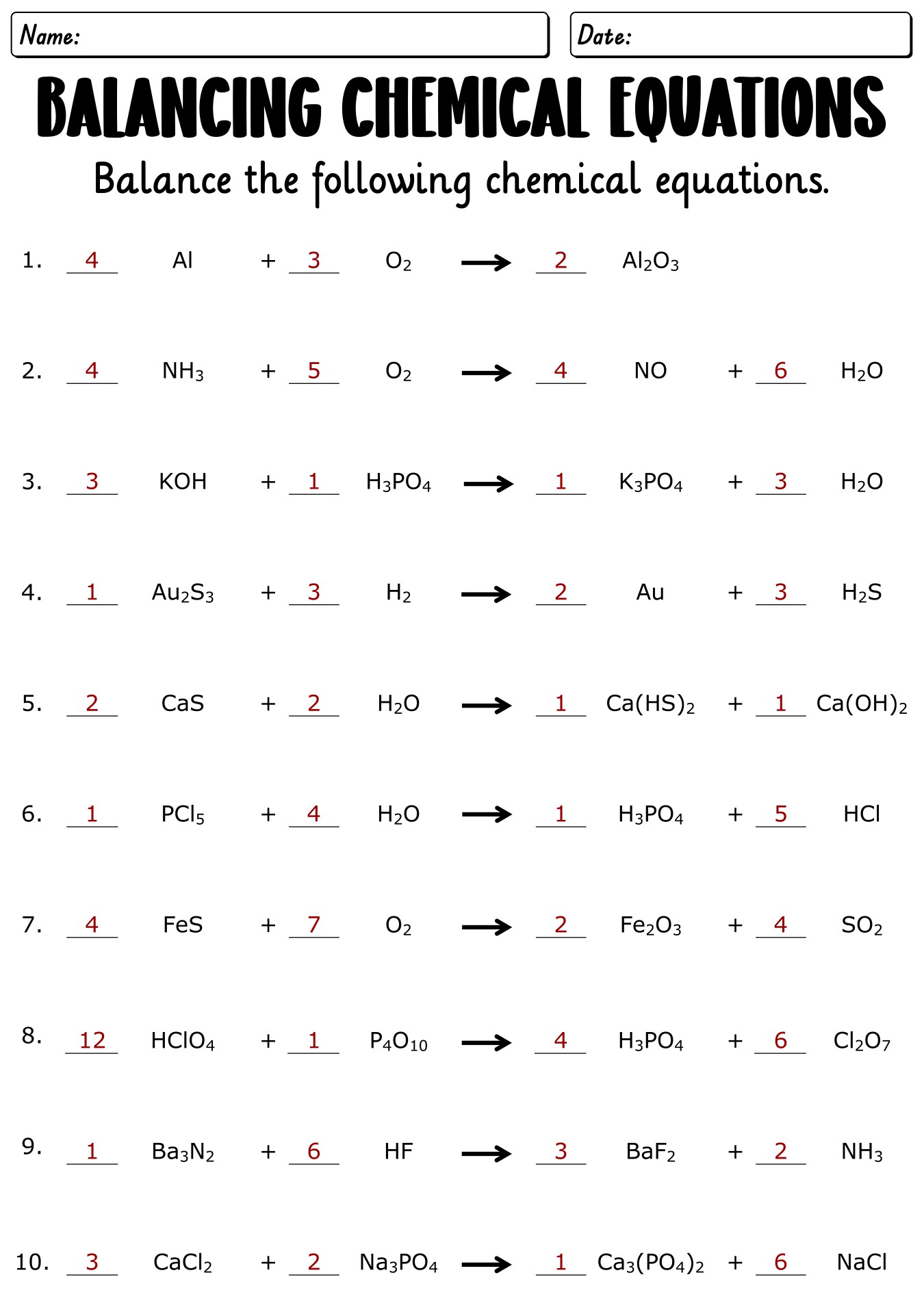
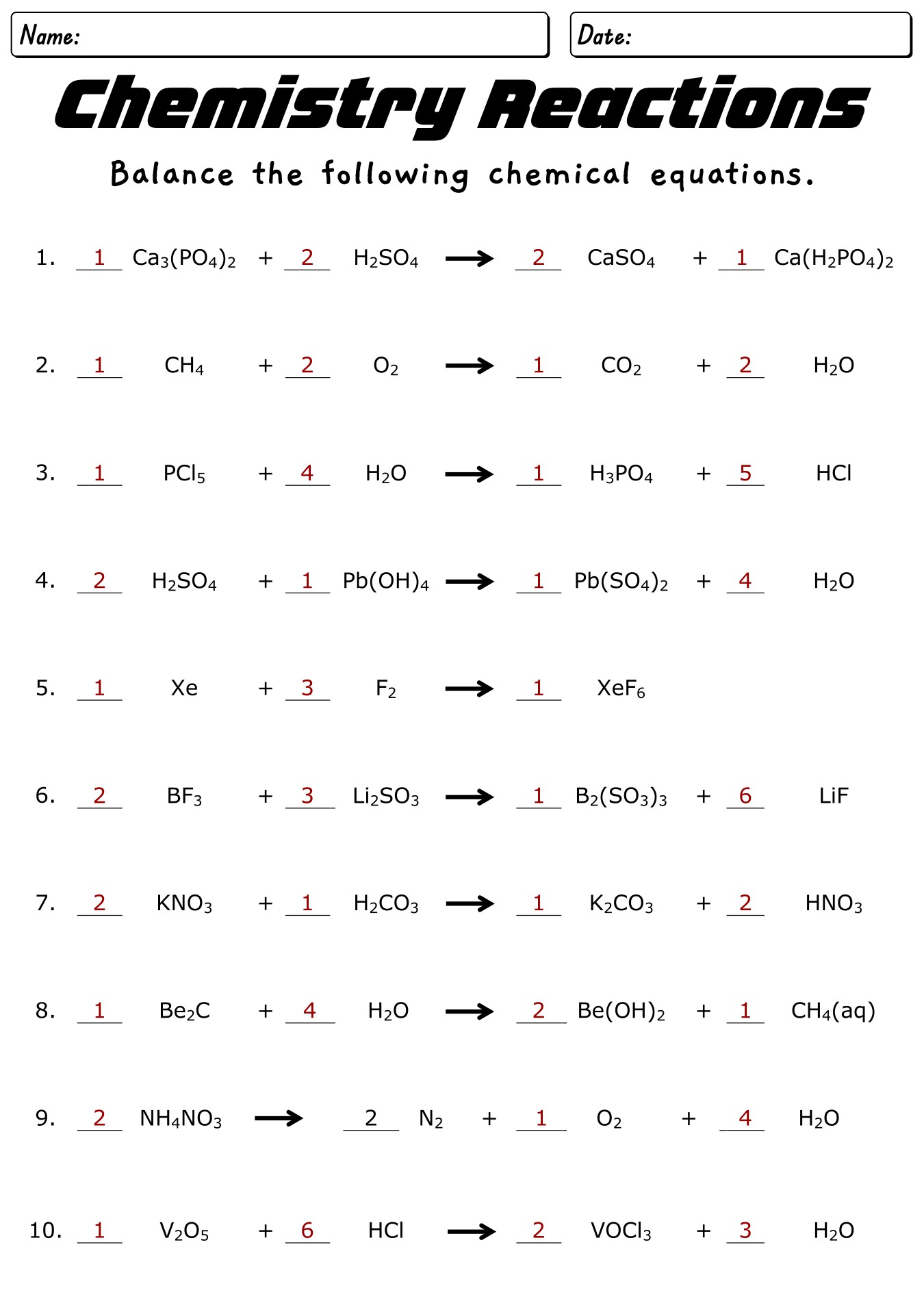
- Chemistry Balancing Equations Worksheet with Key
- Types of Chemical Reactions Worksheet with Answer Guide
- Chemical Reaction Equations Worksheet with Detailed Answers
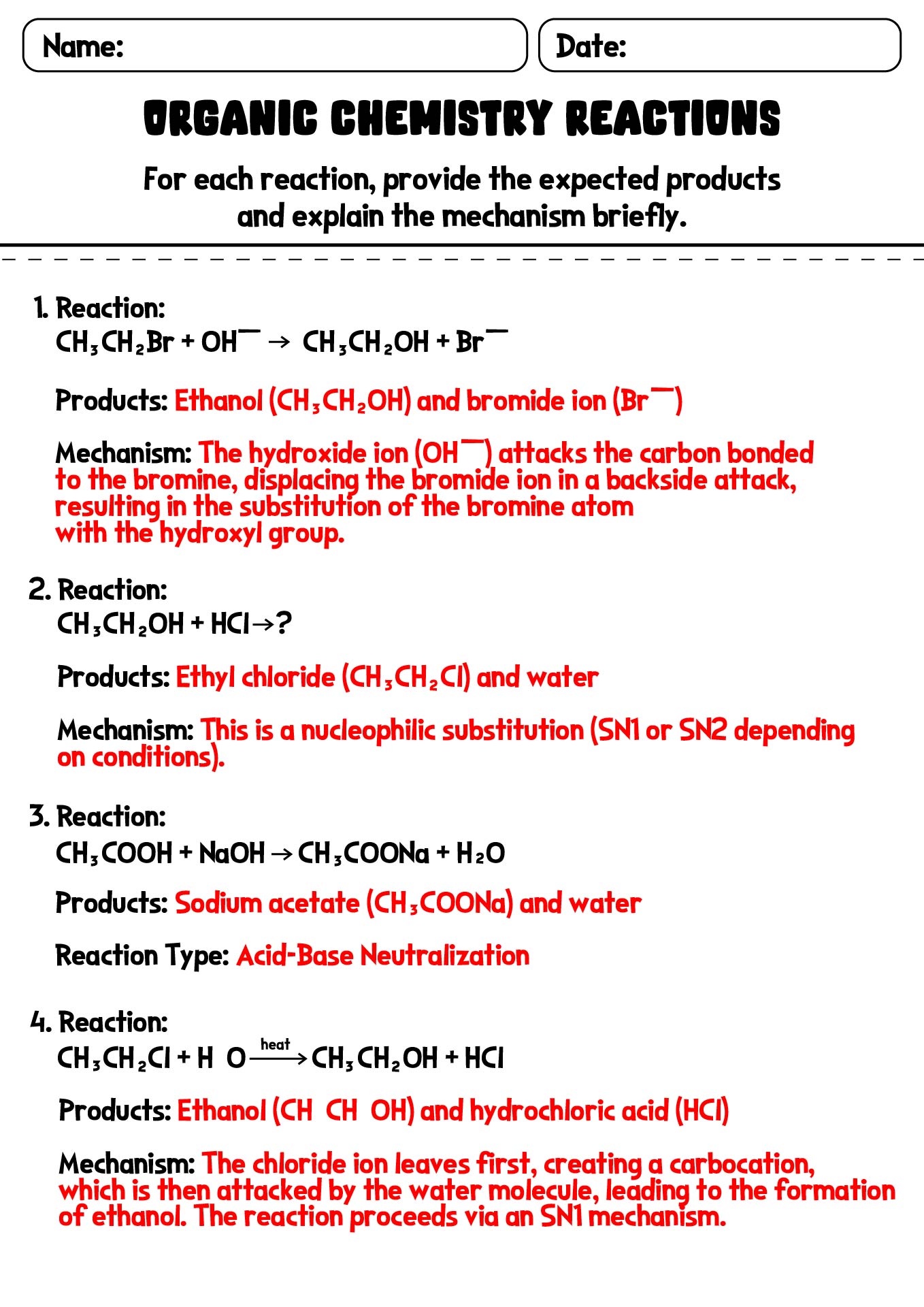
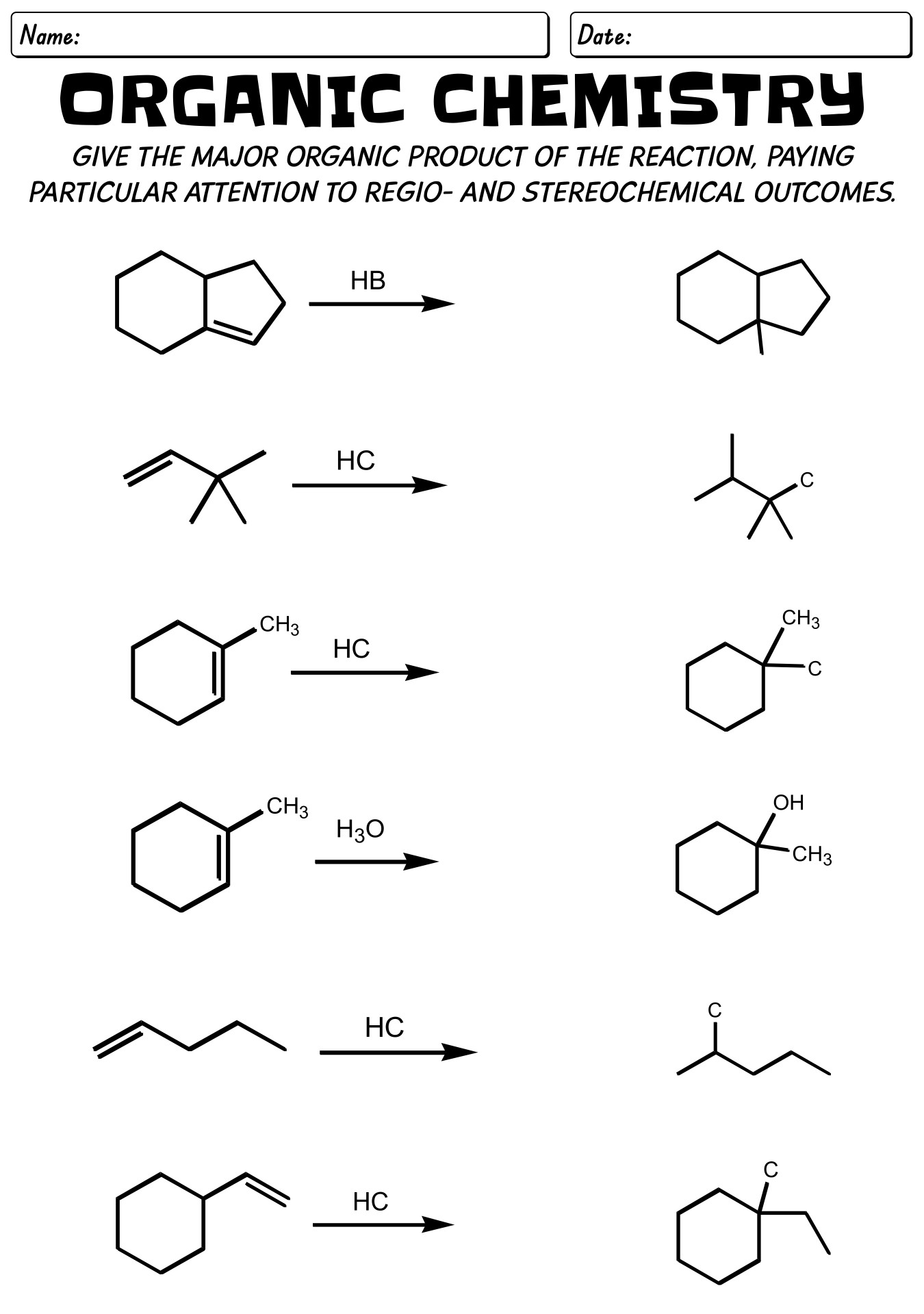
- Organic Chemistry Reactions Worksheet with Answer Sheet
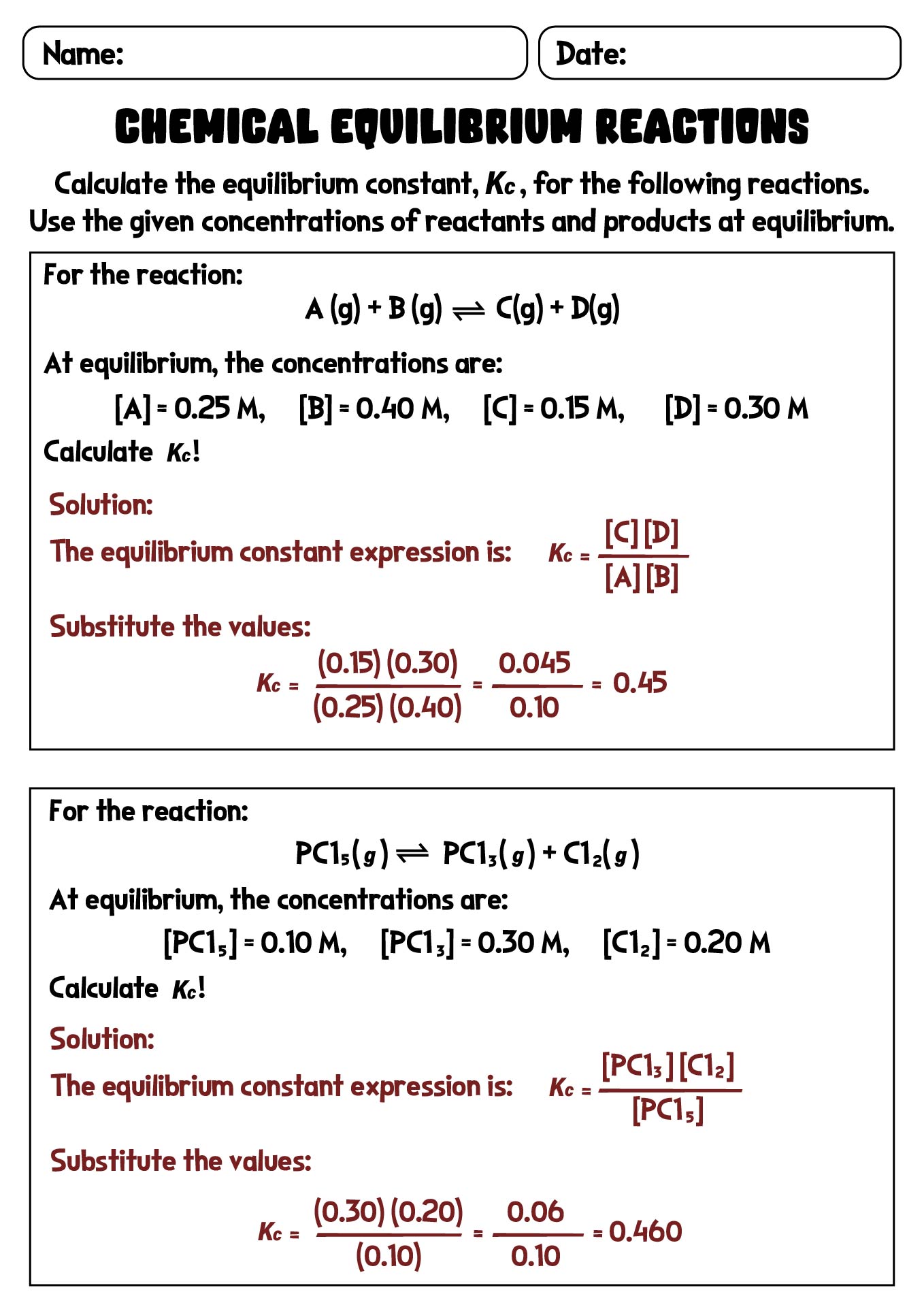
- Chemical Equilibrium Reactions Worksheet with Solutions
- Chemical Equations Balancing Practice Worksheet with Solutions
- Organic Chemistry Reaction Mechanisms Worksheet with Answer Key
- Chemistry Reactions Worksheet with Answers
- High School Chemistry Reactions Worksheet with Answers
- Kinetics and Reaction Rate Worksheets with Answers

More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is a chemical reaction?
A chemical reaction is a process where one or more substances, known as reactants, are converted into different substances, known as products, through the breaking and forming of chemical bonds. This transformation results in the rearrangement of atoms, leading to the creation of new compounds with different properties than the original substances.
What are the reactants in a chemical reaction?
Reactants in a chemical reaction are the substances that interact with each other to undergo a chemical change. They are the starting materials that are consumed in the reaction to produce the products.
What are the products in a chemical reaction?
The products in a chemical reaction are the substances that are formed as a result of the reaction. These products are produced from the reactants, which are the initial substances that undergo a chemical change. The products can be different from the reactants and typically have different properties than the original substances involved in the reaction.
What is the law of conservation of mass?
The law of conservation of mass states that mass cannot be created or destroyed in a chemical reaction, only rearranged into different substances. This means that the total mass of the reactants in a chemical reaction must equal the total mass of the products.
What is meant by an exothermic reaction?
An exothermic reaction is a chemical reaction that releases energy in the form of heat to the surroundings. This means that the products of the reaction have less energy than the reactants, resulting in a decrease in temperature of the surroundings. Examples of exothermic reactions include combustion, neutralization reactions, and many oxidation reactions.
What is meant by an endothermic reaction?
An endothermic reaction is a chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. This means that the energy of the reactants is lower than the energy of the products, and heat is required for the reaction to occur. The reaction feels cold to the touch and is often used for processes that require cooling or as a way to store energy.
What are catalysts and how do they affect chemical reactions?
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy, making it easier for the reaction to occur. This ultimately speeds up the reaction and allows it to proceed more efficiently. Catalysts are important in various industrial processes and biological systems, as they enable reactions to occur at lower temperatures and pressures, reducing energy consumption and costs.
What is meant by a reversible reaction?
A reversible reaction is a chemical reaction that can proceed in both the forward and reverse directions, achieving a state of dynamic equilibrium where the rates of the forward and reverse reactions are equal. This means that reactants can form products, and products can also react to form reactants again. In a reversible reaction, both the forward and reverse reactions occur simultaneously until a balance is reached, allowing the reaction to constantly shift between reactants and products.
What is meant by a redox reaction?
A redox (reduction-oxidation) reaction is a type of chemical reaction in which the oxidation state of atoms is changed. In redox reactions, one substance loses electrons (is oxidized) while another substance gains electrons (is reduced). This transfer of electrons between reactants results in the release or absorption of energy.
What are some factors that can influence the rate of a chemical reaction?
Some factors that can influence the rate of a chemical reaction include the concentration of reactants, temperature, the presence of a catalyst, the surface area of the reactants, and the pressure in the case of gaseous reactions. Additionally, the nature of the reactants, the activation energy required for the reaction to occur, and the orientation of reacting molecules can also impact the rate of a chemical reaction.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments