Binary Ionic Compounds Worksheet
Binary ionic compounds are an essential part of chemistry and understanding how these compounds are formed is crucial for any student studying this subject. Whether you're a high school student preparing for an exam or a college student diving deeper into chemical equations, our Binary Ionic Compounds Worksheet provides the perfect practice to reinforce your knowledge and reinforce the process of writing formulas for these compounds.
Table of Images 👆
- Naming Binary Compounds Worksheet
- Naming Covalent Compounds Worksheet
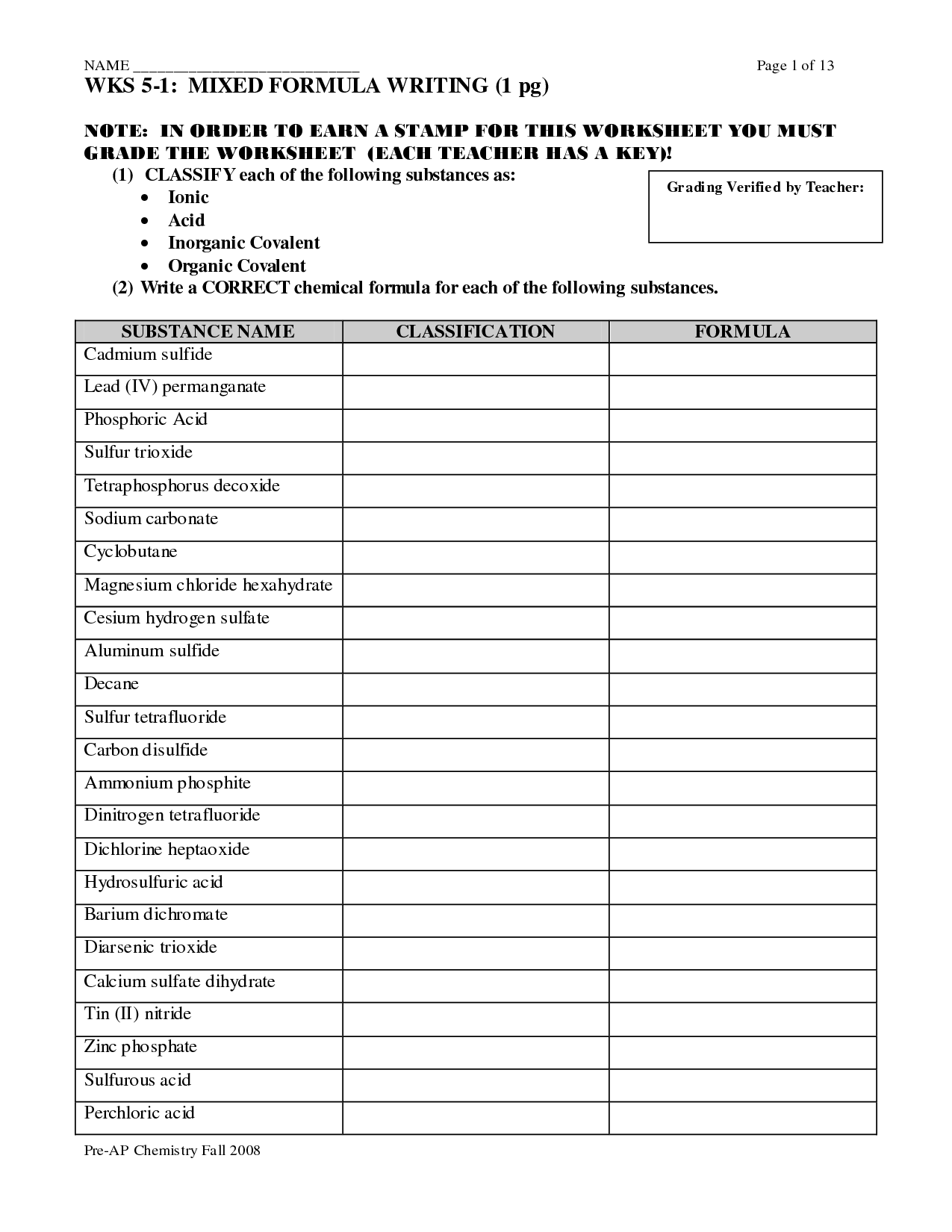
- Binary Ionic Compounds Worksheet 1 Answers
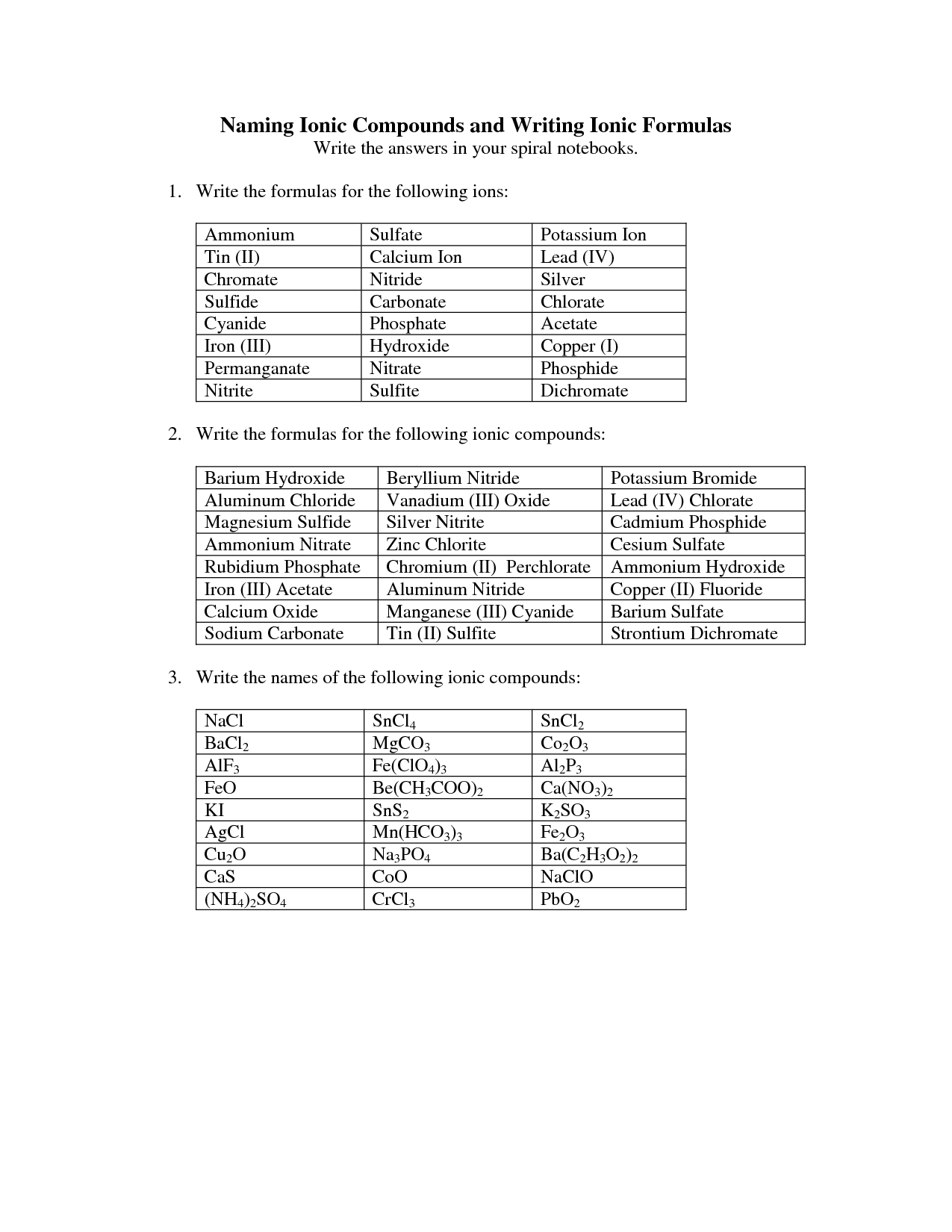
- Naming Ionic Compounds Worksheet
- Writing Ionic Compound Formula Worksheet Answers
- Binary Ionic Compounds Worksheet Answers
- Transition Metal Ionic Compounds Worksheet and Answers
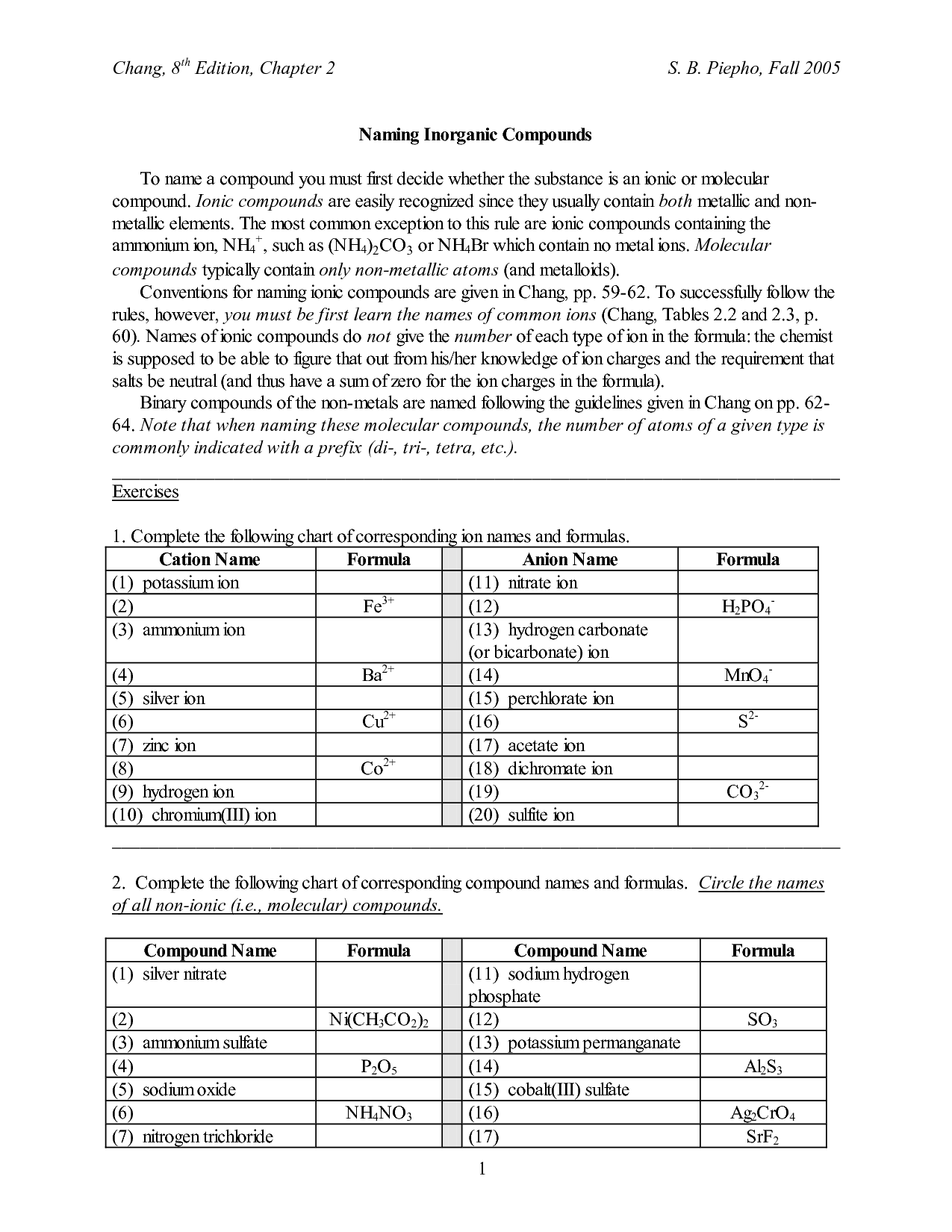
- Naming Ionic Compounds Worksheet Answers
- Naming Ionic Compounds Worksheet Answer Key
- Monatomic and Polyatomic Ion Chart
- Naming Ionic Compounds Worksheet Answer Key
- Ionic Compounds Polyatomic Ions Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is a binary ionic compound?
A binary ionic compound is a type of chemical compound composed of two elements, typically a metal and a nonmetal, where the metal cation and nonmetal anion combine through ionic bonding. The metal element donates electrons to the nonmetal element, resulting in a stable compound with a neutral charge.
How are binary ionic compounds formed?
Binary ionic compounds are formed when a metal atom transfers one or more electrons to a nonmetal atom. The metal atom loses electrons to achieve a stable, full outer electron shell, becoming a positively charged ion. The nonmetal atom gains electrons to fill its outer shell and becomes a negatively charged ion. The opposite charges of the metal cation and nonmetal anion attract each other, resulting in the formation of an ionic bond between the two ions, creating a binary ionic compound.
What is the difference between a cation and an anion?
A cation is a positively charged ion formed when an atom loses one or more electrons, while an anion is a negatively charged ion formed when an atom gains one or more electrons. In other words, cations have more protons than electrons, whereas anions have more electrons than protons.
What is the role of the cation in a binary ionic compound?
The cation in a binary ionic compound is responsible for donating electrons to the anion to form an ionic bond. Cations are positively charged ions that are typically metals and give up electrons to achieve a stable electron configuration. The presence of cations allows for the establishment of electrical neutrality within the compound, as they balance out the negative charge of the anions.
What is the role of the anion in a binary ionic compound?
The anion in a binary ionic compound plays a crucial role in balancing the positive charge of the cation by providing negative charge. Anions are typically non-metal atoms that have gained electrons, resulting in a negative charge. They combine with cations, which are positively charged metal atoms, to form stable compounds through ionic bonding. The anion's role is to stabilize the compound by attracting the cation through electrostatic forces, creating a neutral overall charge in the compound.
Provide an example of a binary ionic compound and its formula.
An example of a binary ionic compound is sodium chloride, which has the chemical formula NaCl. In this compound, sodium (Na) donates an electron to chlorine (Cl), forming an ionic bond between the positively charged sodium cation and the negatively charged chloride anion.
How do you determine the formula of a binary ionic compound given the ions involved?
To determine the formula of a binary ionic compound given the ions involved, you need to balance the charges of the ions by using their charges as subscripts in the formula. The positive ion's charge becomes the subscript of the negative ion, and vice versa. Remove any common factors between the two charges. For example, in the compound formed by sodium ion (Na+) and chloride ion (Cl-), the charges balance at 1:1 to form NaCl. The formula is always written with the positive ion first followed by the negative ion.
What is electrostatic attraction and how does it relate to binary ionic compounds?
Electrostatic attraction refers to the force of attraction between positively and negatively charged particles. In the context of binary ionic compounds, this attraction plays a critical role in holding the compound together. Binary ionic compounds consist of positively charged metal cations and negatively charged nonmetal anions. The electrostatic attraction between these oppositely charged ions is what causes them to form a stable ionic bond, creating a crystal lattice structure in which the positive and negative ions are strongly attracted to each other. This attraction is what gives binary ionic compounds their unique properties, such as high melting and boiling points, as well as their ability to conduct electricity when dissolved in water or melted.
What are some properties of binary ionic compounds?
Binary ionic compounds are composed of metal cations and nonmetal anions that are held together by ionic bonds. They typically have high melting and boiling points due to the strong electrostatic forces between the ions. They are usually soluble in water but not in nonpolar solvents. Binary ionic compounds conduct electricity when dissolved in water or in molten form due to the presence of free-moving ions that can carry electric charge. Additionally, they tend to form crystal structures and exhibit a high degree of symmetry in their arrangement of ions.
How are binary ionic compounds named?
Binary ionic compounds are named by using the name of the cation (metal) followed by the name of the anion (nonmetal) with the ending of the anion changed to "-ide". For example, in the compound NaCl, sodium chloride, "Na" is the cation (sodium) and "Cl" is the anion (chloride), so the compound is named sodium chloride.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments