Basic Atomic Structure Worksheet Answers
Understanding the basic atomic structure is essential for anyone studying chemistry or physics. This worksheet provides clear explanations and answers to help students grasp the fundamental concepts of atoms, subatomic particles, and atomic models. Whether you are a high school student preparing for an exam or a college student needing a quick review, this worksheet is designed to enhance your understanding of the subject.
Table of Images 👆
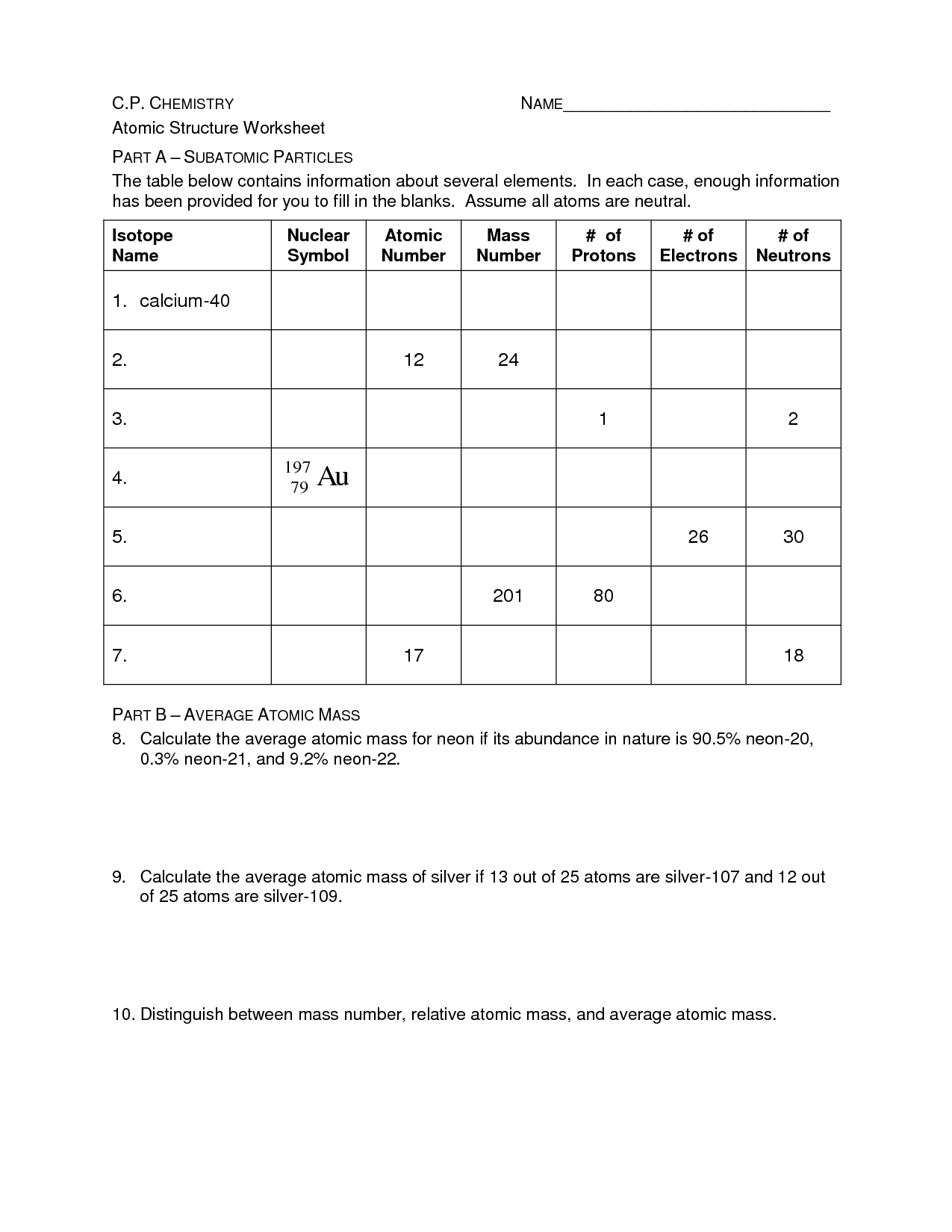
- Basic Atomic Structure Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
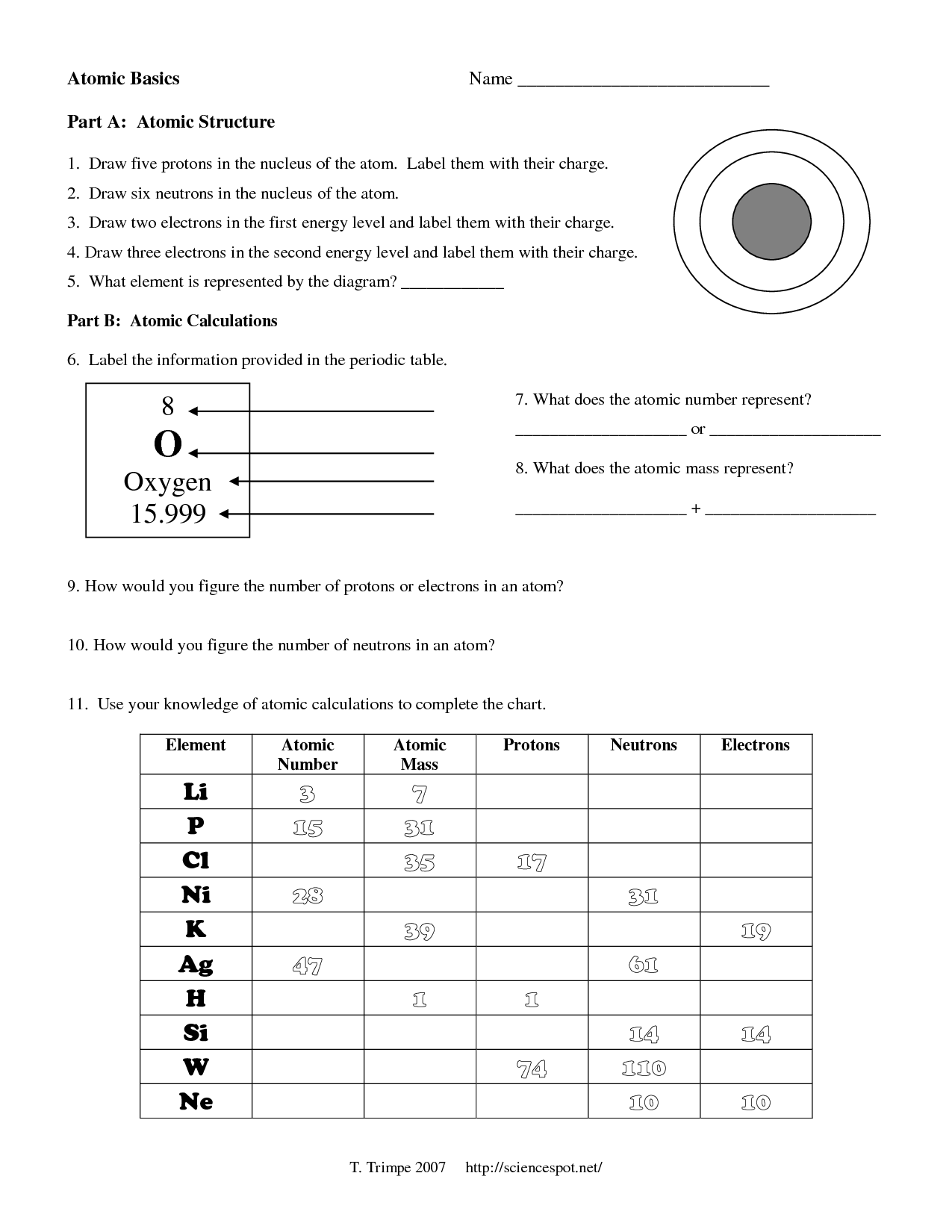
- Atomic Structure Worksheet Answers
- Periodic Table Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
- Atomic Structure Worksheet Answers
- Atomic Structure Worksheet Answers
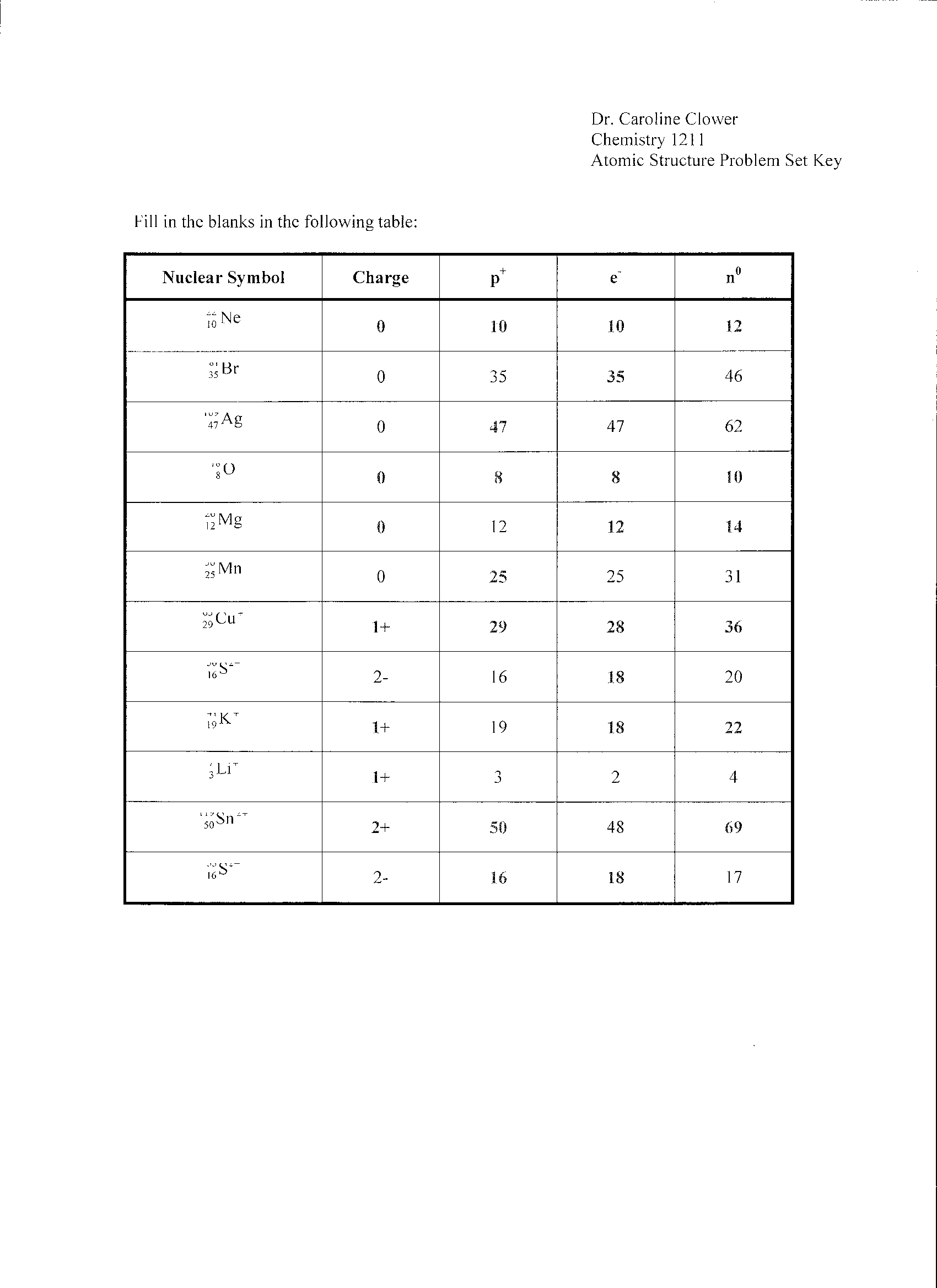
- Isotopes Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
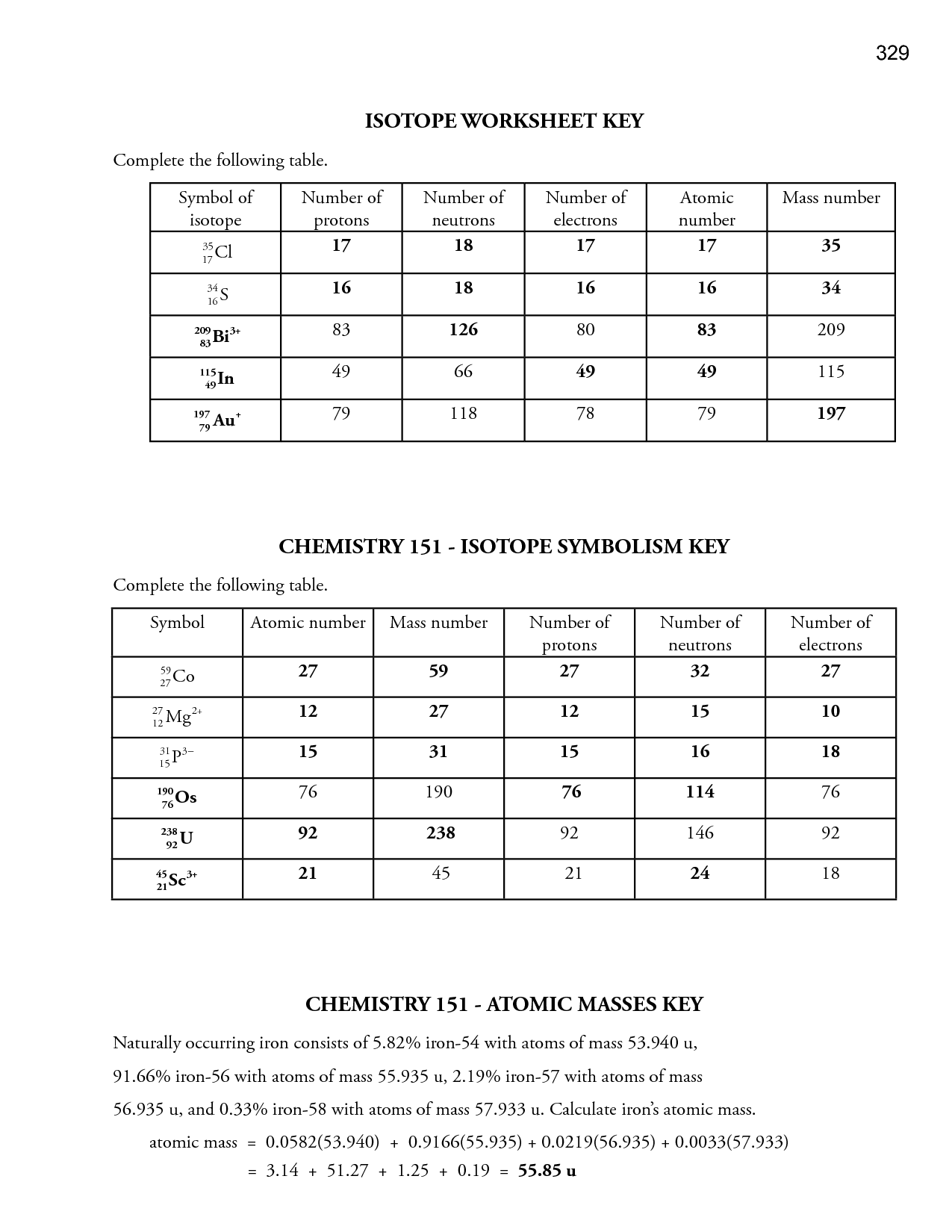
- Atom Structure Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What are atoms?
Atoms are the basic building blocks of matter, consisting of a nucleus made up of protons and neutrons, surrounded by electrons. They are extremely small and are the smallest unit of an element that retains the chemical properties of that element. Atoms combine to form molecules and compounds, and through different arrangements of atoms, they create all the substances in the universe.
What is the atomic nucleus?
The atomic nucleus is the central core of an atom that contains protons and neutrons. It is a tiny, dense region where most of the mass of an atom is concentrated. Protons carry a positive electrical charge while neutrons are neutral, and together they make up the majority of an atom's mass. The nucleus is held together by strong nuclear forces, which are much stronger than the electromagnetic forces that repel positively charged protons.
What are protons?
Protons are positively charged subatomic particles found in the nucleus of an atom. They are one of the three fundamental particles that make up an atom, along with neutrons and electrons. The number of protons in an atom determines its atomic number and defines the element.
What are neutrons?
Neutrons are subatomic particles that are found in the nucleus of an atom, alongside protons. They have no electric charge, which distinguishes them from protons that have a positive charge. Neutrons play a crucial role in determining the stability of an atom as they help bind the protons together through the strong nuclear force.
What is the atomic number?
The atomic number is the number of protons in the nucleus of an atom, which determines its chemical element and its place on the periodic table.
What is the mass number?
The mass number of an atom is the total number of protons and neutrons in its nucleus. It is a whole number and is used to uniquely identify an isotope of an element.
What is an isotope?
An isotope is a variant of a chemical element that has the same number of protons in its nucleus but a different number of neutrons, resulting in a different atomic mass. Isotopes of an element have similar chemical properties but may have varying physical properties, such as stability and radioactive decay rates.
What is the electron cloud?
The electron cloud refers to the area around an atomic nucleus where electrons are likely to be found. It represents the probability distribution of where electrons are likely to be located within an atom. Quantum mechanics describes the electron cloud as a three-dimensional region where there is a high probability of finding electrons based on their energy level and position within the atom.
What are energy levels in an atom?
Energy levels in an atom refer to the specific energies that electrons can have when they are in orbit around the nucleus. Electrons can only exist at certain energy levels within an atom, and they can move between these levels by either absorbing or emitting energy in the form of photons. Each energy level corresponds to a specific distance from the nucleus, with higher energy levels being farther away. The energy levels in an atom are quantized, meaning they can only have specific, discrete values rather than continuous values.
What is the difference between an electron's energy level and its energy state?
An electron's energy level refers to the specific amount of energy it possesses, typically represented by quantum numbers in an atom's electron configuration. On the other hand, an electron's energy state relates to its overall condition, which can encompass not only its energy level but also factors such as its position and spin within an atom. In essence, while an energy level pertains to a precise quantity of energy, an energy state describes the electron's holistic situation within an atomic system.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments