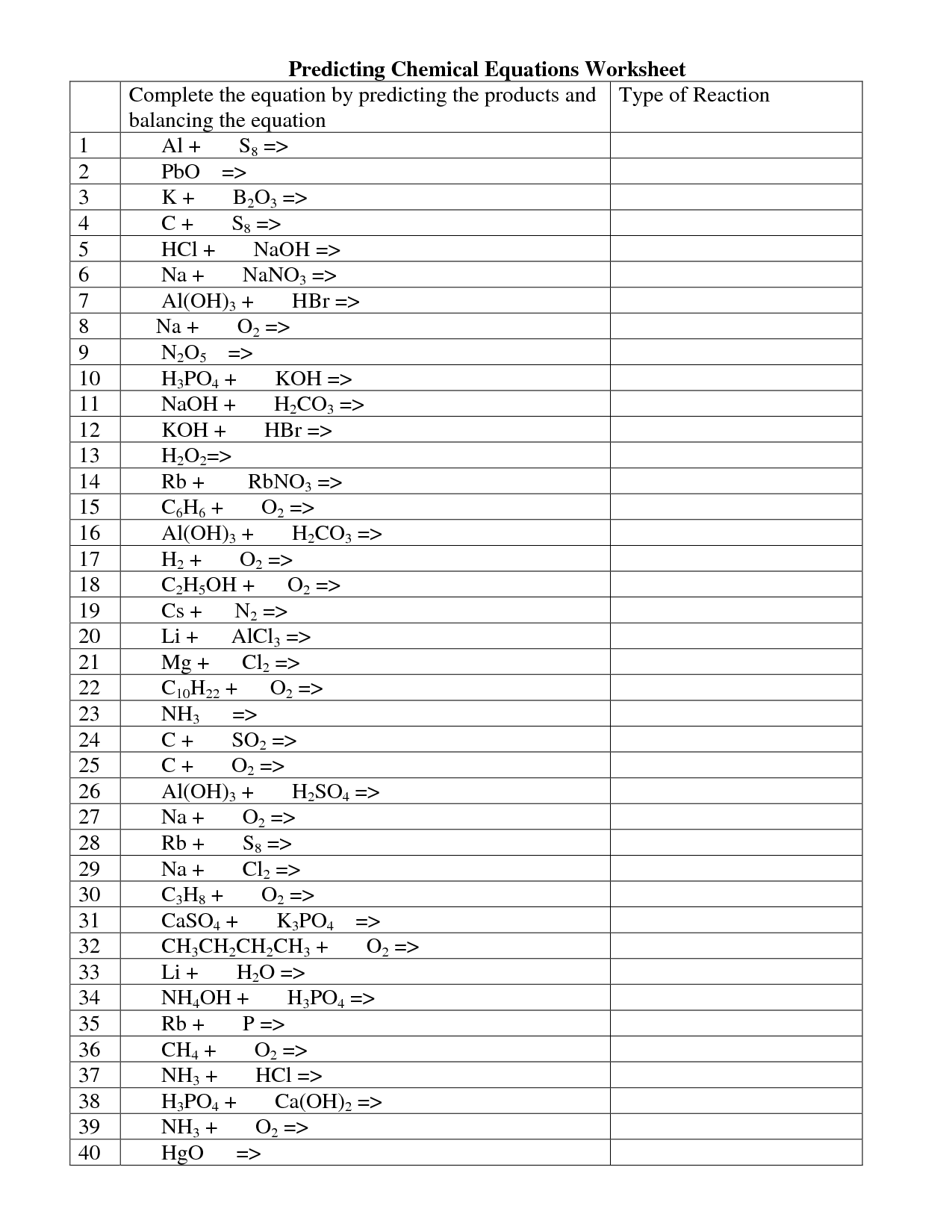
Balancing Chemical Equations Worksheet
Are you struggling to balance chemical equations? If so, you're not alone. Many students find this task challenging. But don't worry, help is at hand! We have created a balancing chemical equations worksheet that is designed to make this process easier for you. By providing you with a variety of equations to work on, this worksheet will give you the practice you need to master this important skill.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is a chemical equation?
A chemical equation is a symbolic representation of a chemical reaction using chemical formulas and symbols to show the reactants and products involved in the reaction, as well as the conservation of mass and charge. It provides information on the substances that interact in a reaction and the substances that are produced as a result of the reaction.
What is the purpose of balancing a chemical equation?
The purpose of balancing a chemical equation is to ensure that the law of conservation of mass is upheld. By balancing the equation, the number of atoms of each element on the reactant side is equal to the number of atoms of the same element on the product side. This helps to show the exact quantities of each substance involved in a chemical reaction and ensures that no atoms are created or destroyed during the process.
How do you identify the reactants and products in a chemical equation?
In a chemical equation, reactants are written on the left side of the arrow and products are written on the right side. Reactants are the substances that undergo chemical reactions and are transformed into products. The arrow indicates the direction of the reaction, showing the conversion of reactants into products. Reactants are typically listed first, followed by the products, making it easy to identify them in the equation.
What is the law of conservation of mass and how does it relate to balancing equations?
The law of conservation of mass states that in a chemical reaction, matter is neither created nor destroyed, only rearranged. This means that the total mass of the reactants must be equal to the total mass of the products. When balancing chemical equations, we ensure that the number of atoms of each element is the same on both sides of the equation. This process ensures that the law of conservation of mass is obeyed, as the total mass before and after the reaction remains constant.
What are coefficients and how are they used to balance equations?
Coefficients in a chemical equation represent the ratio of moles of each substance involved in the reaction. They are used to balance equations by adjusting the coefficients of the reactants and products to ensure that the same number of atoms of each element are present on both sides of the equation. This balancing is necessary to obey the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction, only rearranged.
What is the difference between a balanced and unbalanced chemical equation?
A balanced chemical equation has an equal number of atoms of each element on both sides of the reaction arrow, ensuring that the law of conservation of mass is followed. An unbalanced chemical equation, on the other hand, does not have the same number of atoms of each element on both sides of the reaction arrow, which means mass is not conserved. Balancing the equation involves adjusting the coefficients of the reactants and products to achieve equality in the number of atoms of each element.
How do you determine the correct coefficients to balance an equation?
To determine the correct coefficients to balance an equation, you need to ensure that the number of atoms of each element is the same on both sides of the equation. Start by balancing the atoms that appear in only one reactant and one product. Then, adjust the coefficients of the remaining substances to balance the equation. You can use trial and error while following the law of conservation of mass as a guide to find the correct coefficients that balance the equation.
Can you balance an equation by adjusting the subscripts of the chemical formula?
No, balancing an equation by adjusting subscripts is not allowed as it changes the identity of the chemical species involved in the reaction. Balancing an equation is done by adding coefficients to the chemical formulas to ensure that the number of atoms on both sides of the equation is equal.
What are some common strategies for balancing complex chemical equations?
Some common strategies for balancing complex chemical equations include starting with the most complex or least abundant element, adjusting coefficients to balance the number of atoms of each element on both sides of the equation, using fractions when necessary, and checking that the equation is balanced by counting the number of atoms of each element on both sides. Additionally, practice and experience can help improve efficiency and speed in balancing equations.
What are the steps to follow when balancing a chemical equation?
To balance a chemical equation, start by writing down the chemical formula for the reactants and products. Then, count the number of each type of atom on both sides of the equation and use coefficients to balance the equation by adjusting the number of molecules of each compound. Begin by balancing the most complex and uncommon compounds, and then work on the more common elements. Ensure that the number of atoms of each element is the same on both sides of the equation, making sure to only adjust coefficients and not subscripts. Finally, double-check to ensure that the equation is balanced by counting the number of each type of atom on both sides.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments