Balancing Chemical Equations Worksheet Answer Key 1-15
Chemical equations can be a challenging subject for many students, but fear not! Look no further as we bring you a comprehensive and reliable answer key for balancing chemical equations on worksheets. Whether you are a high school student or someone studying chemistry at a higher level, these answer keys will provide you with the necessary guidance and help you grasp the concepts of chemical equations in a structured and organized manner.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Answers
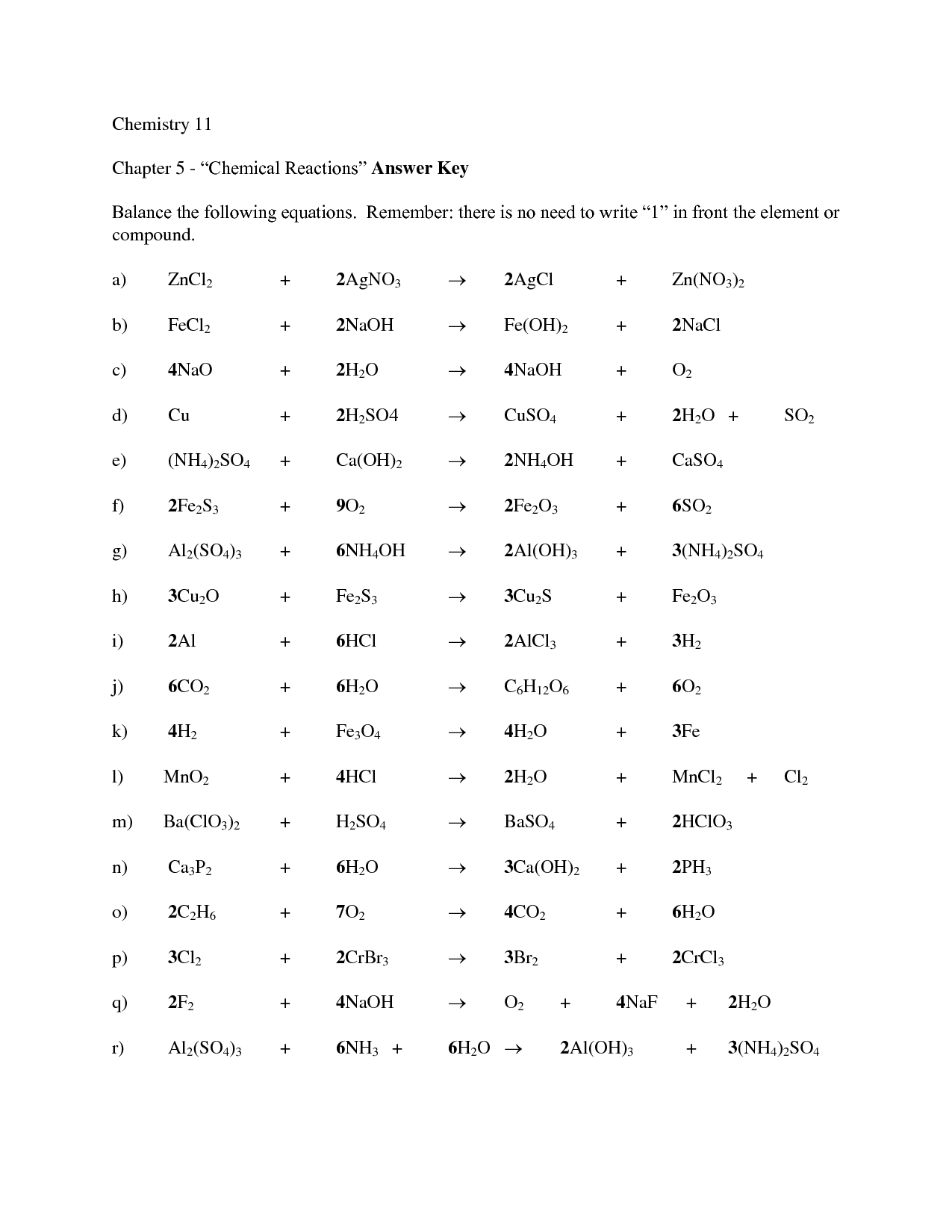
- Balancing Chemical Equations Answer Key
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Key
- Balancing Chemical Equations Answer Key
- Balancing Chemical Equations Worksheet Answer Key
- Writing Chemical Equations Worksheet Answers Balancing
- Balancing Chemical Equations Worksheet Answer Key
- Balancing Chemical Equations Worksheet Answer Key
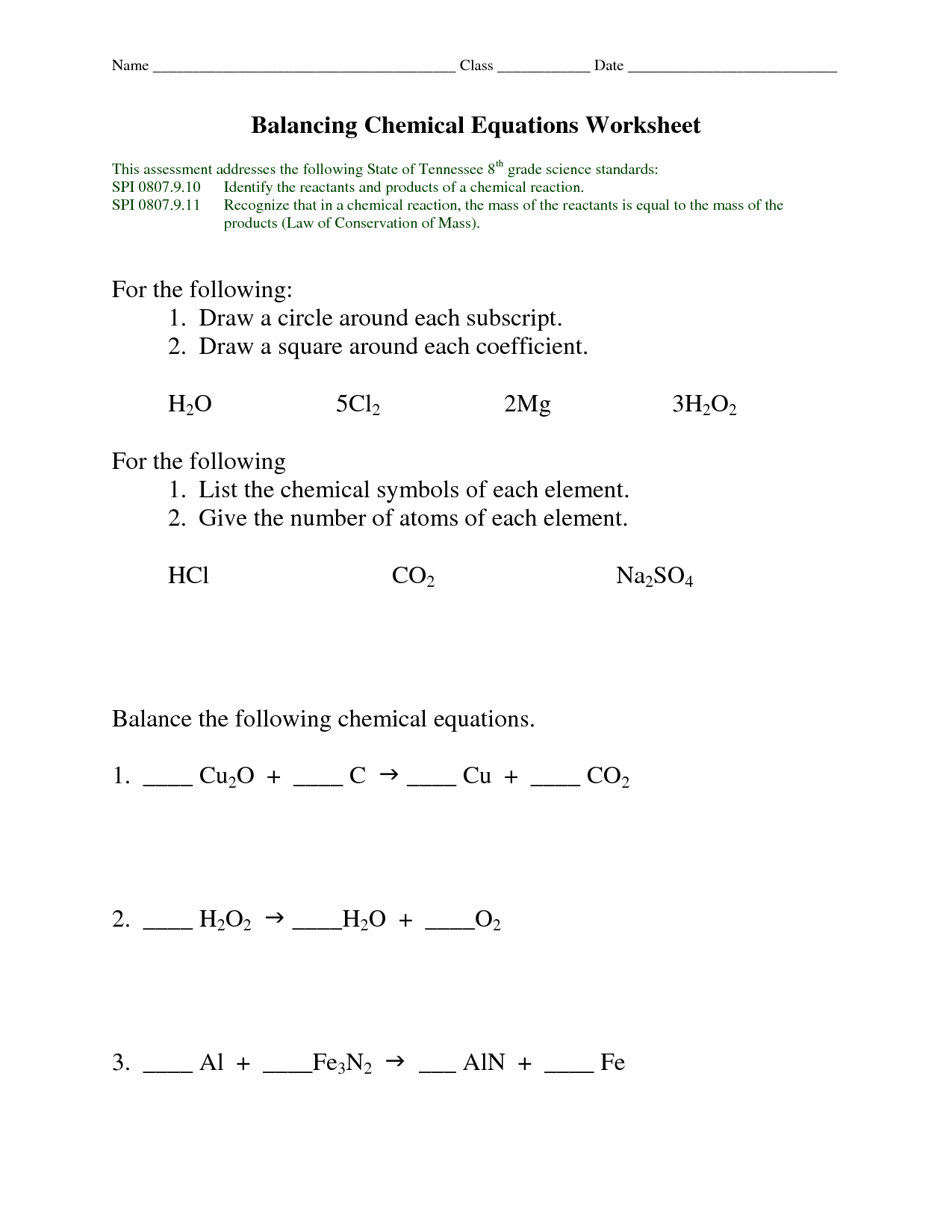
- Balancing Chemical Equations Worksheet
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet 1 Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
What is a chemical equation?
A chemical equation is a symbolic representation of a chemical reaction, showing the reactants on the left side and the products on the right side, with the chemical formulas of the substances involved and the coefficients representing the ratio of molecules or moles.
How are chemical equations balanced?
Chemical equations are balanced by adjusting the coefficients of the reactants and products so that the number of atoms of each element is the same on both sides of the equation. This is done by using stoichiometric coefficients and following the law of conservation of mass, ensuring that no atoms are gained or lost during the reaction. The goal is to have the same number of each type of atom on both sides of the equation to accurately represent the chemical reaction taking place.
Why is it important to balance chemical equations?
Balancing chemical equations is important because it represents the law of conservation of mass, which states that the total mass of the elements present before a chemical reaction must be equal to the total mass of the elements after the reaction. By balancing the equation, it ensures that there is an equal number of atoms for each element on both sides of the reaction, providing a clear understanding of the reactants and products involved in the reaction.
What is the meaning of a coefficient in a chemical equation?
A coefficient in a chemical equation represents the relative number of moles of each reactant and product involved in a reaction. It is a numerical factor that is placed in front of a molecule or formula to balance the equation, ensuring that the law of conservation of mass is obeyed. Coefficients are used to indicate the ratio in which substances react and are produced in a chemical reaction.
What is the purpose of subscripts in a chemical equation?
Subscripts in a chemical equation are used to indicate the number of atoms of each element present in a molecule. They are important for accurately representing the composition of compounds and balancing chemical equations to ensure that the law of conservation of mass is obeyed.
How do you identify the reactants and products in a chemical equation?
In a chemical equation, reactants are the substances that are present at the beginning of a chemical reaction and undergo a change, while products are the substances that are produced as a result of the chemical reaction. Reactants are typically listed on the left side of the equation, separated by a plus sign, and products are listed on the right side, separated by an arrow pointing towards the products. The reactants react with each other to form the products, and the conservation of mass principle ensures that the total mass of reactants must equal the total mass of products.
What is the law of conservation of mass and how does it relate to balancing equations?
The law of conservation of mass states that matter cannot be created or destroyed in chemical reactions, only rearranged. This means that the total mass of the reactants must be equal to the total mass of the products. When balancing chemical equations, this principle is applied by ensuring that the number of atoms in the reactants equals the number of atoms in the products. This is done by adjusting coefficients in front of the chemical formulas to make the amounts of each element on both sides of the equation equal, thus following the law of conservation of mass.
How do you balance equations that involve polyatomic ions?
To balance equations involving polyatomic ions, treat the polyatomic ion as a single unit and balance it as you would an element. Ensure the total charge on each side of the equation is equal, as polyatomic ions carry a specific charge. Remember to adjust coefficients for both the compounds and the polyatomic ions, if needed, to achieve overall charge balance and equal numbers of each type of atom on both sides of the equation. Keep practicing this process to become more comfortable with balancing equations involving polyatomic ions.
What are some tips or strategies for balancing more complex chemical equations?
One tip for balancing more complex chemical equations is to start by balancing the atoms of elements that appear only once on each side of the equation. Then, focus on balancing the atoms of oxygen and hydrogen last, as these elements commonly appear in multiple compounds. Utilizing coefficients to balance the equation can also help, making sure to adjust the coefficients as needed to maintain the balance of all elements. Additionally, practicing regularly with different chemical equations can improve your proficiency in balancing complex equations efficiently.
Is there a specific order or sequence to follow when balancing equations?
Yes, when balancing chemical equations, it is typically best to start by balancing the atoms that appear in only one reactant and one product. Next, balance the atoms that appear in multiple compounds on each side of the equation, remembering to adjust coefficients to ensure the same number of each type of atom on both sides. Finally, double check your work to make sure the equation is fully balanced, with the same number of each type of atom and the conservation of mass maintained.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments