Atomic Spectra Worksheet
Are you a student studying atomic spectra and looking for a helpful resource to reinforce your knowledge? Look no further! We have just the solution for you - a comprehensive atomic spectra worksheet that will enhance your understanding of this fascinating subject.
Table of Images 👆
- Electromagnetic Spectrum Worksheet Answers
- Atomic Model Timeline Worksheet
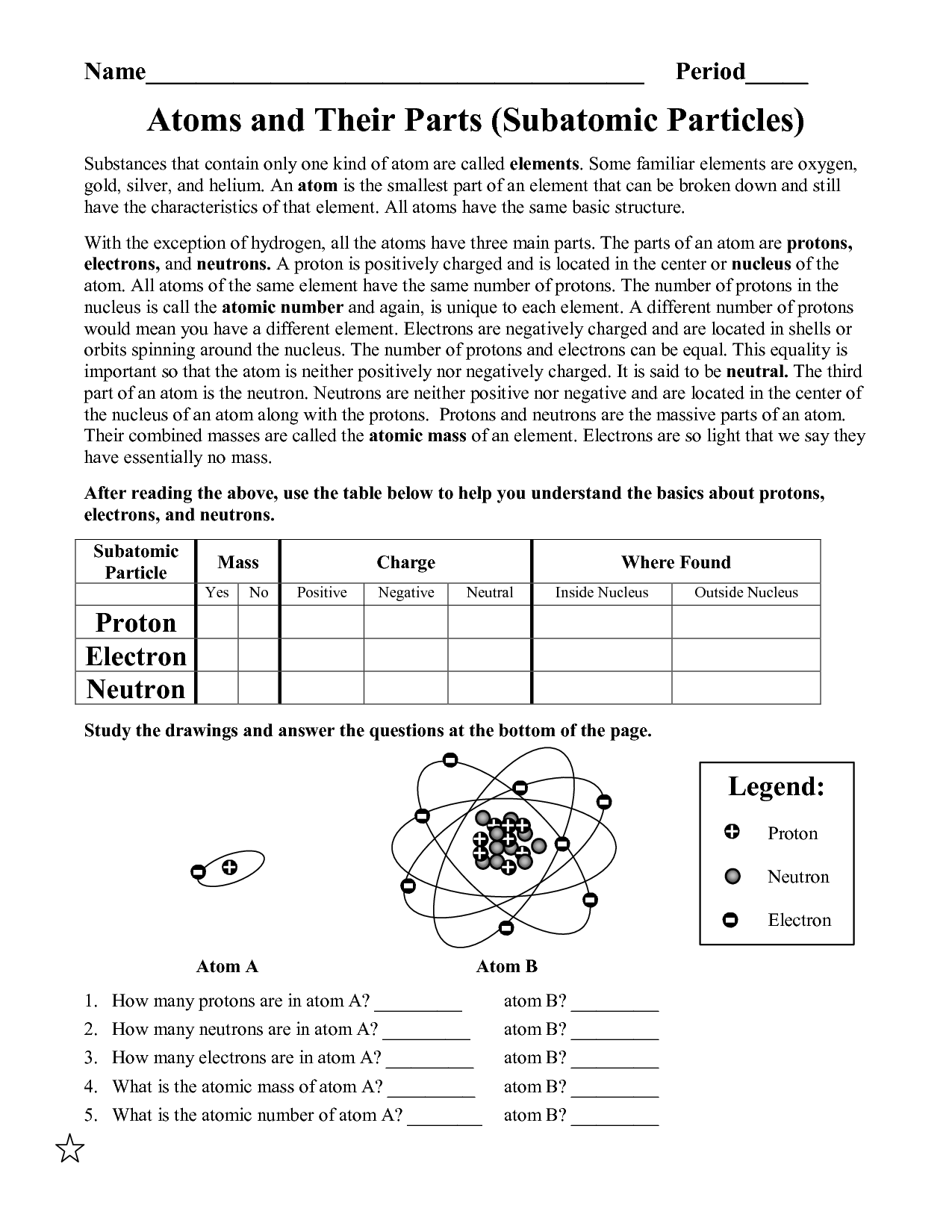
- Basic Atomic Structure Worksheet Answer Key
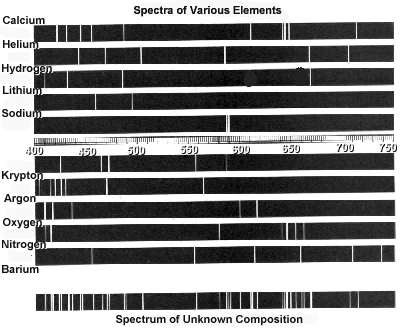
- Element Emission Spectra Worksheet
- Emission Line Spectrum Worksheet
- Bohr Atomic Models Worksheet Answers
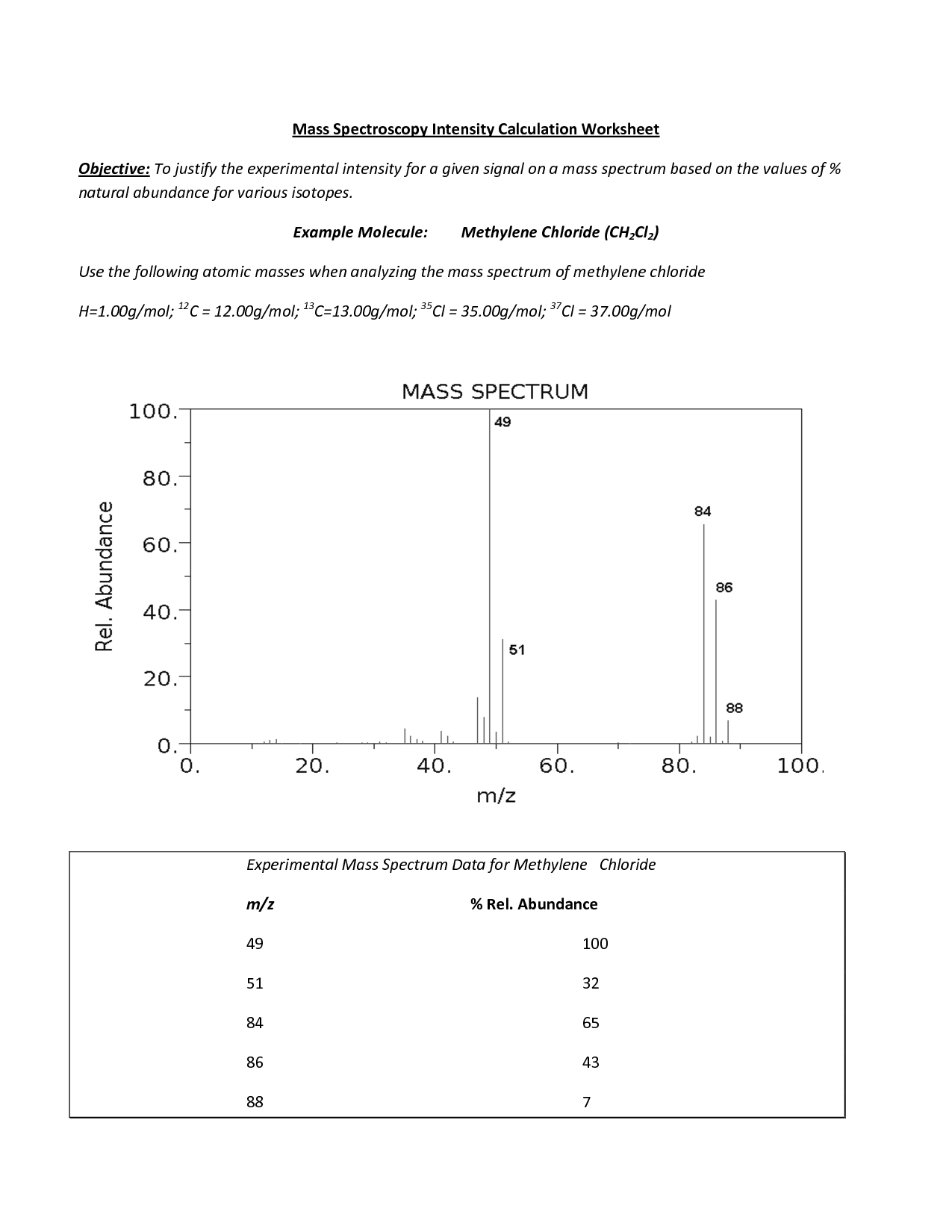
- Atomic Mass Calculations Worksheet
- Atomic Model Worksheet Answers
- Atoms Family Worksheet
- Quantum Mechanics Worksheet
- Atomic Bomb Worksheets Answers
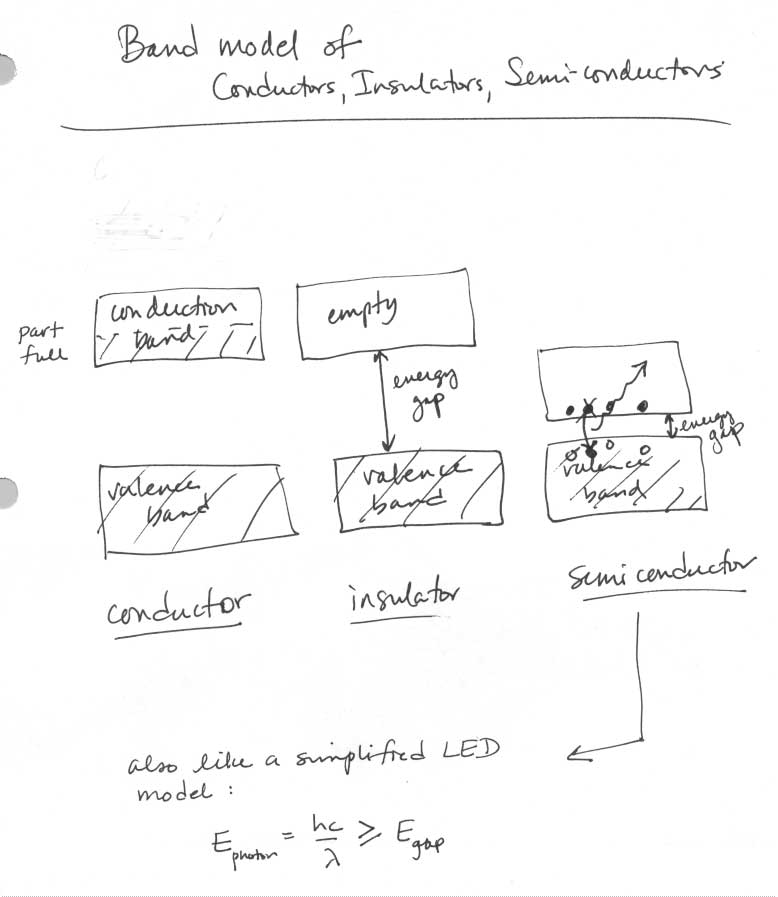
- Conductors and Insulators Worksheet
- Electromagnetic Spectrum Worksheet Answers
- Atoms Protons Neutrons Electrons Worksheet
- Bohr Atomic Models Worksheet Answers
- Light and Atoms Worksheet 2 Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is atomic spectroscopy?
Atomic spectroscopy is a technique used to analyze the elemental composition of a sample by measuring the characteristic wavelengths of light emitted or absorbed by atoms when they undergo transitions between energy levels. This method allows scientists to identify and quantify the presence of different elements in a sample, making it a valuable tool in various fields such as environmental analysis, food safety, and materials science.
How does atomic spectroscopy work?
Atomic spectroscopy works by first vaporizing a sample containing the element of interest, then exposing it to a specific wavelength of light, typically from a light source such as a hollow cathode lamp or a laser. When the element absorbs this light, electrons in its atoms get excited to higher energy levels. As the electrons return to their original energy levels, they emit light at characteristic wavelengths, which are detected by a spectrophotometer. By analyzing the emitted light, the concentration of the element in the sample can be determined based on the intensity of the emitted radiation, allowing for qualitative and quantitative analysis of elements in a sample.
What are the different types of atomic spectra?
The different types of atomic spectra are continuous spectra, emission spectra, and absorption spectra. Continuous spectra consist of a continuous range of colors or wavelengths and are produced by hot, dense objects. Emission spectra arise when atoms emit light at specific wavelengths as electrons move from higher to lower energy levels. Absorption spectra occur when atoms absorb specific wavelengths of light, resulting in dark lines superimposed on a continuous spectrum, as seen when light passes through a cool, dilute gas.
What information can be determined from an atomic spectrum?
An atomic spectrum can provide information about the energy levels of electrons in an atom. It can reveal the specific wavelengths of light that are emitted or absorbed when electrons move between these energy levels. By analyzing the spectrum, scientists can determine the elemental composition of a sample, as each element has a unique set of energy levels and corresponding transitions that produce distinct spectral lines. This information is crucial for identifying elements in various samples and studying their electronic structure.
How are absorption and emission spectra different?
Absorption spectra show the absorption of specific wavelengths of light by a substance, indicating the energy levels of the substance's electrons, while emission spectra show the emission of specific wavelengths of light by a substance when excited, revealing the specific energy transitions of the substance's electrons as they return to lower energy levels. In essence, absorption spectra show what wavelengths are absorbed by a substance, whereas emission spectra show what wavelengths are emitted by a substance.
How are energy levels and atomic spectra related?
Energy levels in an atom play a crucial role in determining the atomic spectra. When an atom absorbs energy, its electrons move to higher energy levels. Subsequently, when these excited electrons fall back to lower energy levels, they release the absorbed energy in the form of electromagnetic radiation, resulting in emission or absorption lines in the atomic spectra. The specific transitions between different energy levels give rise to distinct spectral lines, allowing scientists to identify elements based on their unique atomic spectra patterns.
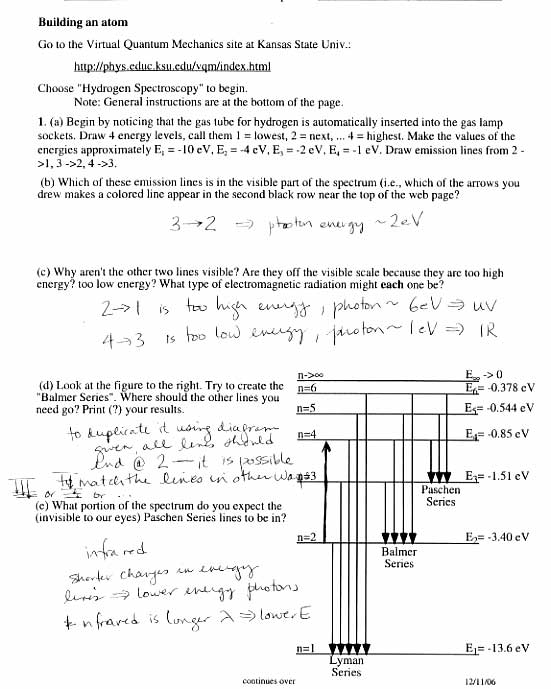
What is the significance of the Balmer series in atomic spectra?
The Balmer series is significant in atomic spectra because it represents the wavelengths of light emitted and absorbed by hydrogen atoms when electrons undergo transitions between energy levels. This series was one of the first spectral line series observed and played a crucial role in the development of quantum mechanics as it provided experimental evidence for the quantization of electron energy levels in atoms. The Balmer series also helped in accurately determining the Rydberg constant and understanding the structure of atomic spectra, laying the foundation for modern atomic and quantum physics.
How do electrons transition between energy levels in atomic spectra?
Electrons transition between energy levels in atomic spectra by gaining or losing discrete amounts of energy in the form of photons. When an electron moves to a higher energy level, it absorbs a photon of specific energy, causing it to reach an excited state. Conversely, when the electron moves to a lower energy level, it emits a photon with corresponding energy, transitioning back to a stable state. These transitions are governed by quantum mechanics and are responsible for the unique spectral lines observed in atomic spectra.
What is the relationship between atomic spectra and elements?
Atomic spectra are unique patterns of light emitted or absorbed by atoms when their electrons move from one energy level to another. Each element has a distinct atomic structure with characteristic energy levels, leading to specific atomic spectra. By analyzing these spectra, scientists can identify elements based on the light they emit or absorb, demonstrating a direct relationship between atomic spectra and the elements they represent.
How is atomic spectra used in various fields, such as chemistry and astronomy?
Atomic spectra are crucial in both chemistry and astronomy for identification and analysis purposes. In chemistry, atomic spectra can help identify elements present in a sample by matching the observed spectral lines with known patterns of different elements. This is essential for determining the composition of compounds and mixtures. In astronomy, atomic spectra are used to study the composition and temperature of stars, galaxies, and other celestial objects by analyzing the unique spectral lines emitted or absorbed by various elements. This information helps astronomers understand the properties and behaviors of objects in space, providing insights into the universe's composition and evolution.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments