Atom Worksheets with Answer Keys
If you are an educator or a parent looking for atom worksheets that come with answer keys, you've come to the right place. Worksheets are an effective tool for teaching and reinforcing concepts, and having a corresponding answer key can help ensure accuracy and provide guidance. In this blog post, we will explore a variety of atom worksheets that cover different aspects of this subject, along with their corresponding answer keys.
Table of Images 👆
- Atoms Ions and Isotopes Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
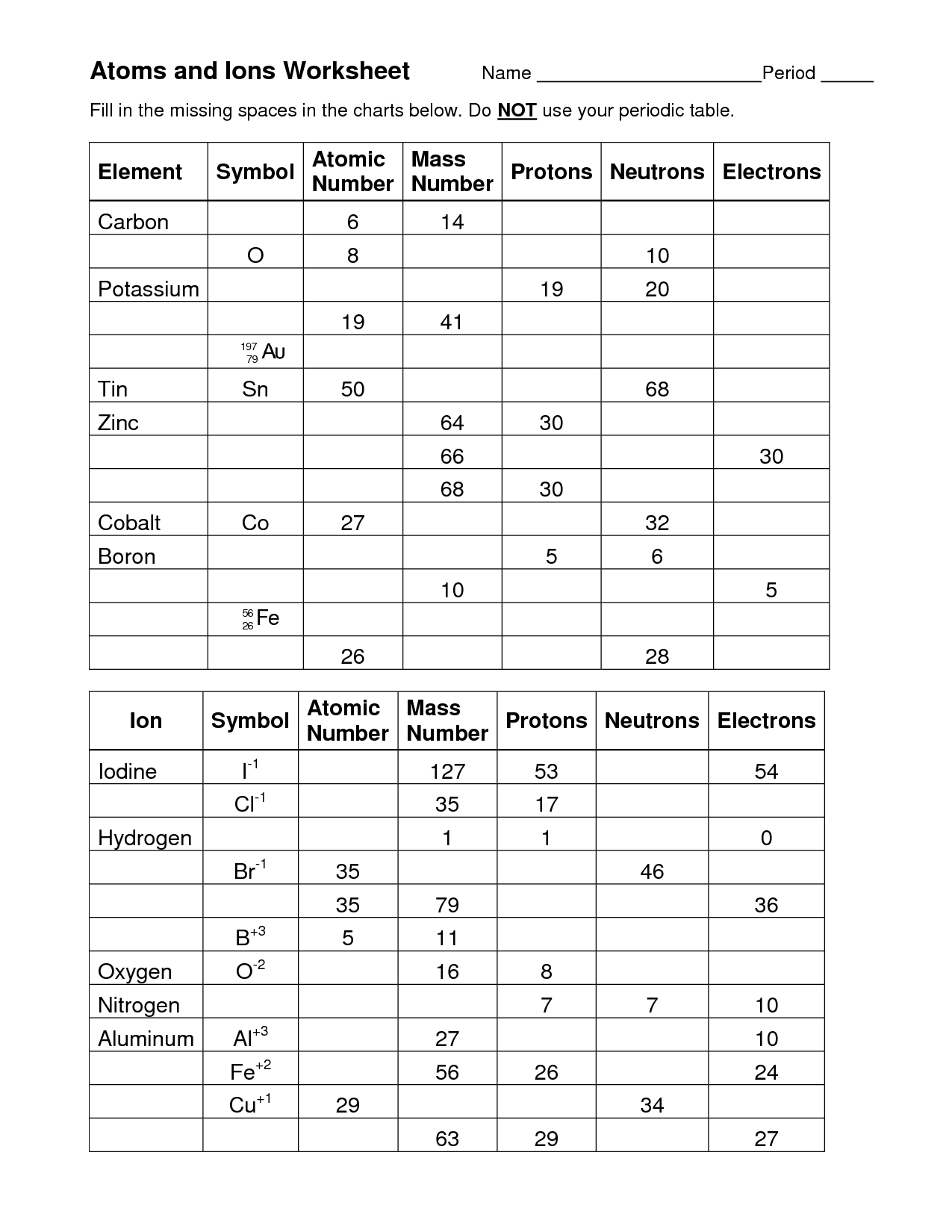
- Atoms and Ions Worksheet Answer Key
- Stoichiometry Worksheet Answer Key
- Basic Atomic Structure Worksheet Answer Key
- Electrons in Atoms Worksheet Answers
- Atoms and Ions Worksheet Answers
- Atomic Structure of an Atom Worksheet
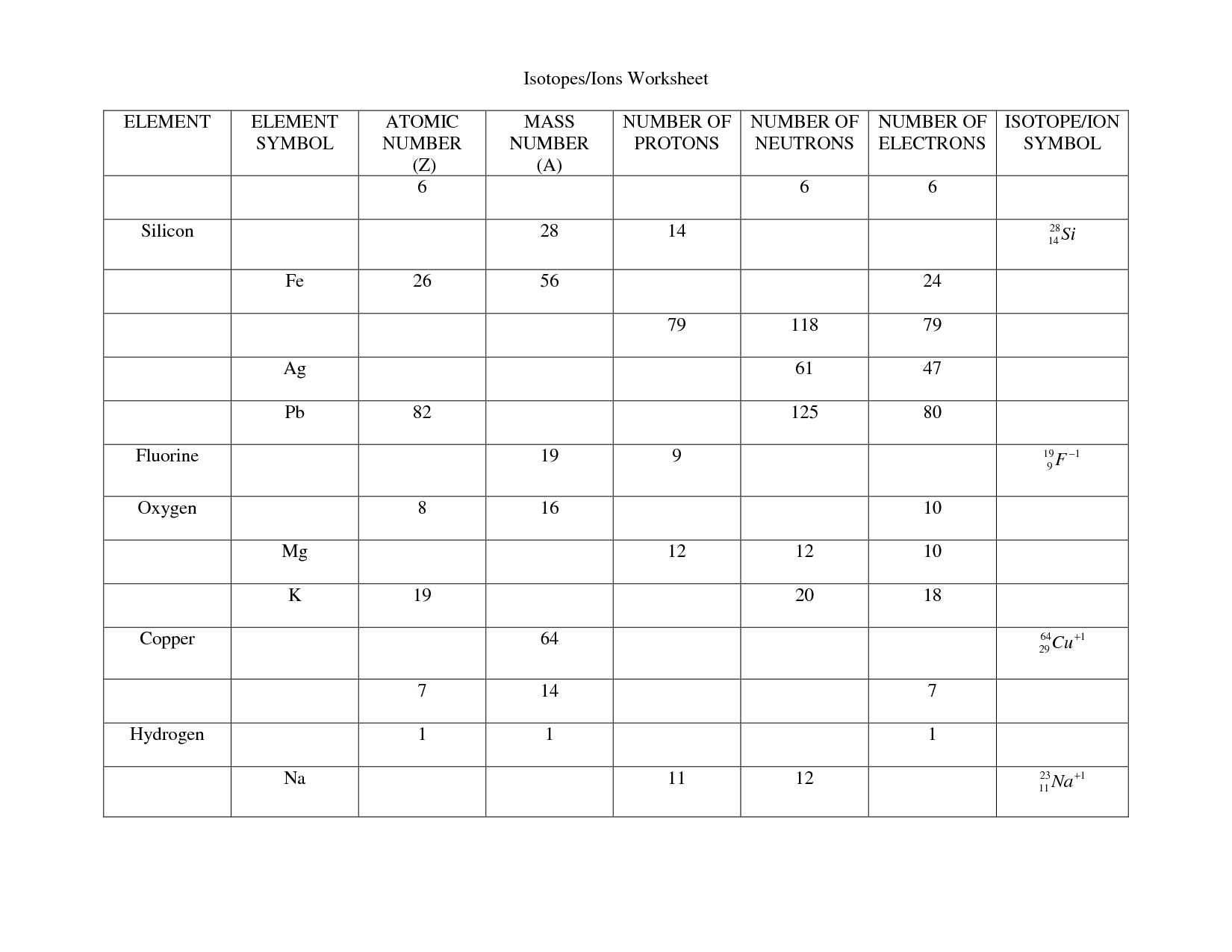
- Atoms Isotopes and Ions Worksheet Answers
- Atoms Family Worksheet Answers
- Mass and Atomic Number Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is an atom worksheet?
An atom worksheet is a learning tool that typically contains questions, diagrams, and exercises related to the structure and properties of atoms. It is designed to help students understand the basic components of an atom, such as protons, neutrons, and electrons, as well as concepts like atomic number, atomic mass, and isotopes. By completing an atom worksheet, students can practice their knowledge and skills in atomic theory and enhance their understanding of how atoms interact and form molecules.
What are the basic components of an atom?
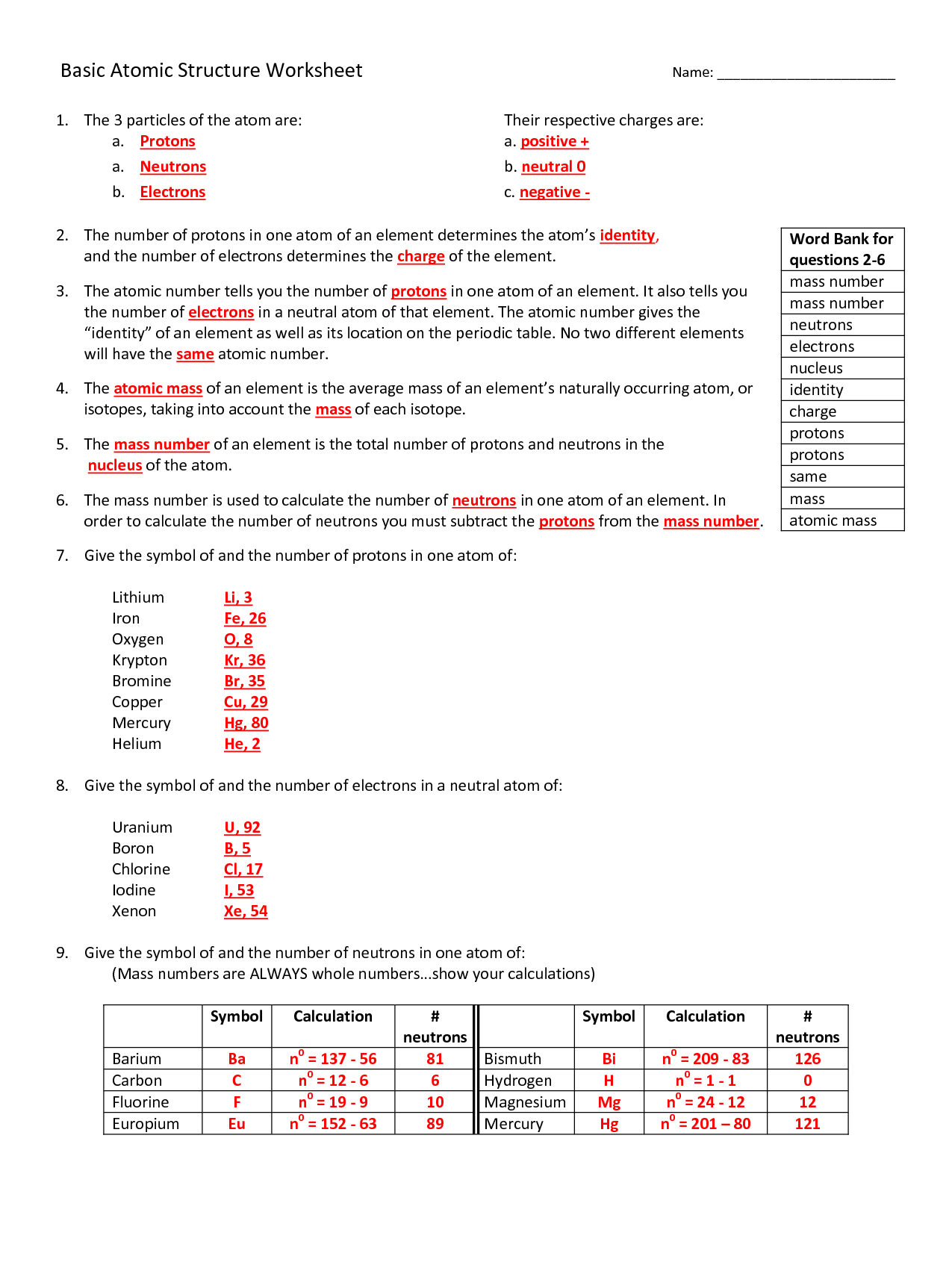
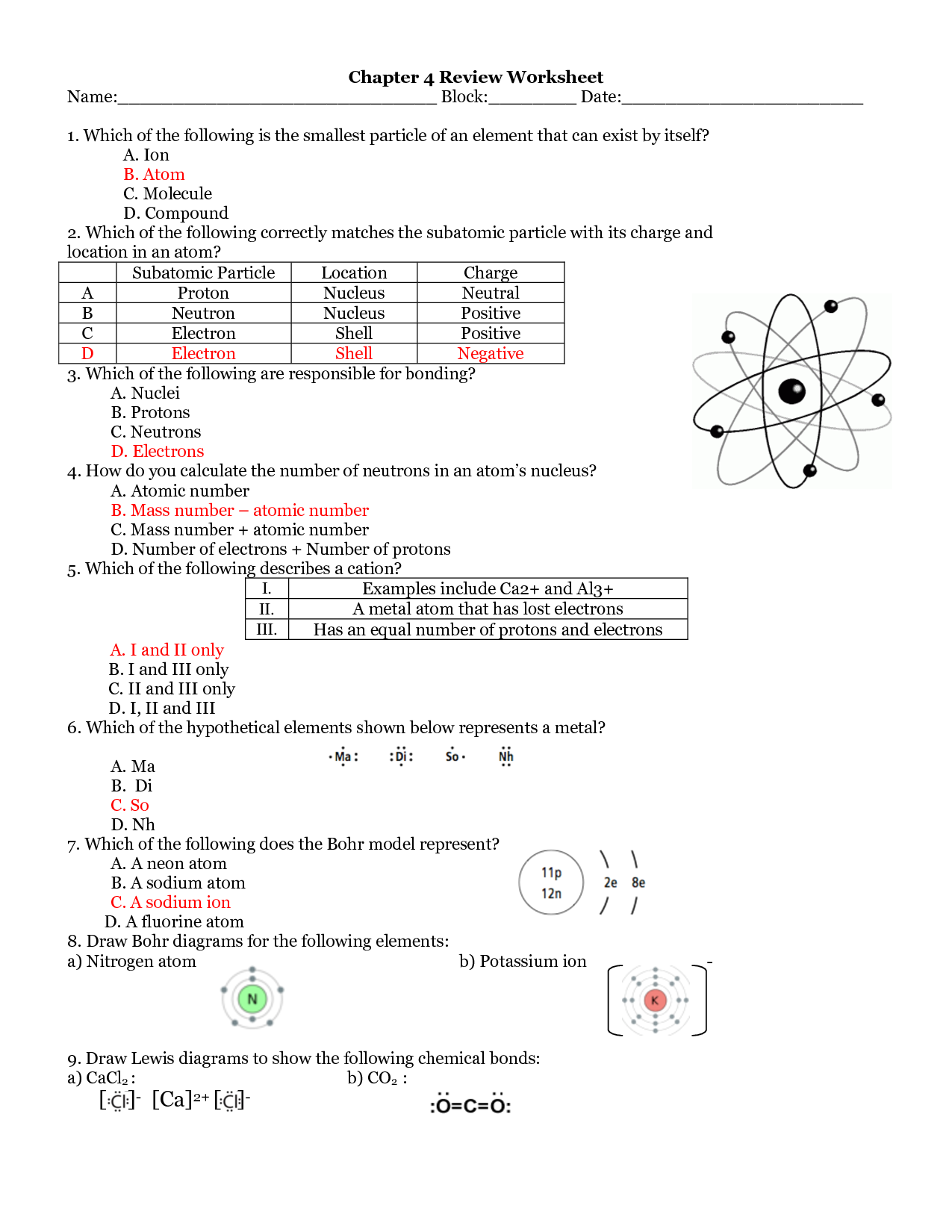
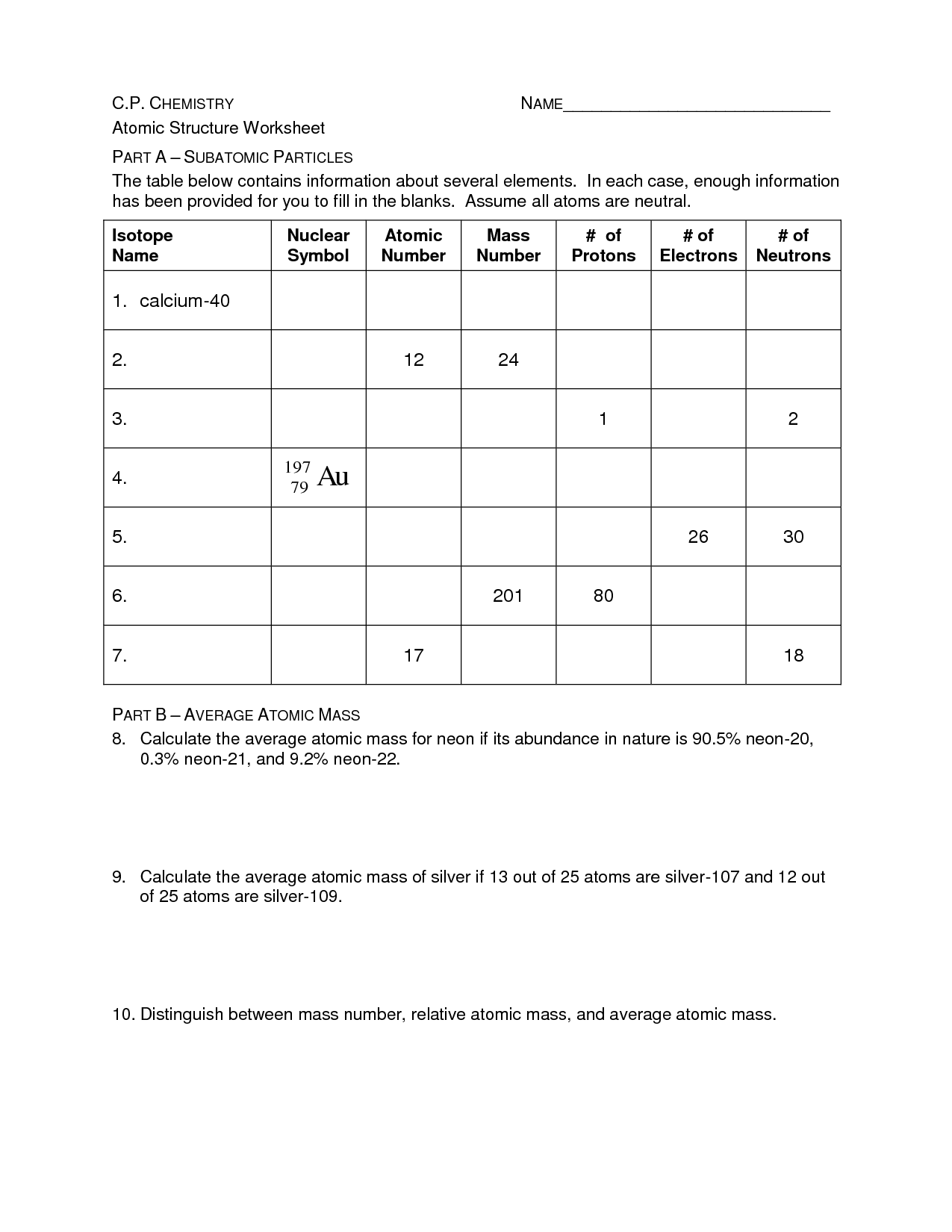
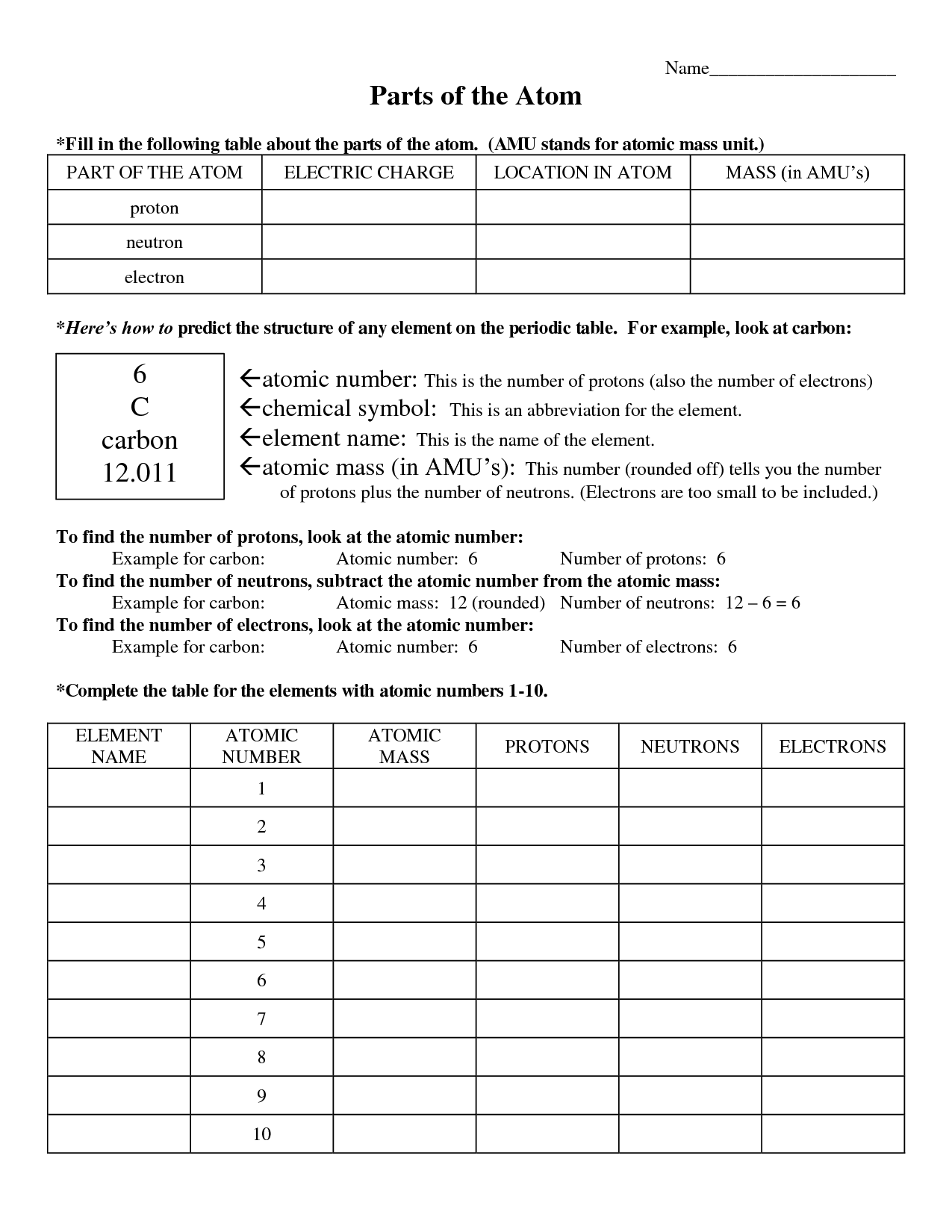
The basic components of an atom are protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit around the nucleus in various energy levels.
How are protons and neutrons different from electrons?
Protons and neutrons are located in the nucleus of an atom, while electrons orbit the nucleus. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. Protons and neutrons contribute to the mass of an atom, while electrons have negligible mass. In summary, protons and neutrons are found in the nucleus, have mass, and have a charge, while electrons are located in the electron cloud surrounding the nucleus, have very little mass, and have a negative charge.
What is the atomic number of an atom?
The atomic number of an atom is the number of protons found in the nucleus of the atom.
How is the atomic mass of an atom determined?
The atomic mass of an atom is determined by adding together the number of protons and neutrons in its nucleus. Protons and neutrons have a relative atomic mass of 1, so by summing these together, we can calculate the atomic mass of the atom. The number of electrons orbiting the nucleus is not included in the atomic mass calculation as their mass is considered negligible compared to protons and neutrons.
What is an isotope?
An isotope is a variant of an element that has the same number of protons but a different number of neutrons in its nucleus, leading to variations in atomic mass. This results in isotopes having the same chemical properties but slightly different physical properties, such as stability and radioactive decay.
How are electron shells or energy levels organized?
Electron shells or energy levels are organized in concentric layers around the nucleus of an atom, with each shell representing a specific energy level. The shells are labeled as K, L, M, N, and so on, with increasing distance from the nucleus. Electrons fill these shells starting from the innermost shell (closest to the nucleus) before moving to the outer shells, following the Aufbau principle and the Pauli exclusion principle to ensure stability and the proper arrangement of electrons within an atom.
What are valence electrons?
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are involved in chemical bonding and are responsible for determining the reactivity and stability of an atom. The number of valence electrons dictates an element's position in the periodic table and influences its behavior in chemical reactions.
How do atoms bond with each other?
Atoms bond with each other through interactions between their electrons. In chemical bonding, atoms can share electrons (covalent bonding), transfer electrons (ionic bonding), or simply attract each other due to differences in electronegativity (polar covalent bonding). These interactions create stable arrangements of atoms called molecules or compounds.
What is the significance of the periodic table in understanding atoms?
The periodic table is significant in understanding atoms because it organizes elements based on their chemical properties and atomic structure. It helps to predict the behavior of elements and their compounds, as elements in the same group have similar properties. The table also provides key information such as atomic number, atomic mass, and electron configuration, which are essential in understanding the characteristics and reactivity of atoms. Additionally, the periodic table allows scientists to study trends in properties across periods and groups, leading to a better comprehension of atomic structure and the relationships between different elements.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments