5th Grade Science Mixtures and Solutions Worksheets
Are you a 5th grade science teacher looking for engaging and effective worksheets to reinforce your students' understanding of mixtures and solutions? Look no further! In this blog post, we will explore a range of carefully crafted worksheets focusing on this challenging topic. These worksheets are designed to cater to the specific needs of 5th graders, ensuring that they grasp important concepts related to mixtures and solutions through targeted practice and meaningful exercises.
Table of Images 👆
- Mixtures and Solutions Worksheets
- 5th Grade Science Mixtures and Solutions
- Solutions and Mixtures Worksheet 5th Grade
- Solutions and Mixtures Worksheet 5th Grade
- Mixtures and Solutions Worksheets
- Mixtures and Solutions Worksheets
- 5th Grade Mixtures and Solutions Activities
- Mixtures and Solutions Study Guide Answers
- Separating Mixtures Worksheet
- Separation Techniques Worksheet
- 5th Grade Science Mixtures and Solutions Practice Sheets

More Science Worksheets
6 Grade Science WorksheetsScience Heat Energy Worksheets with Answer
Science Worksheets Light and Sound
1st Grade Life Science Worksheets
7th Grade Science Cells Worksheets
Worksheets Life Science Vocabulary
8th Grade Science Scientific Method Worksheet
Science Worksheets All Cells
5th Grade Science Mixtures and Solutions Worksheets
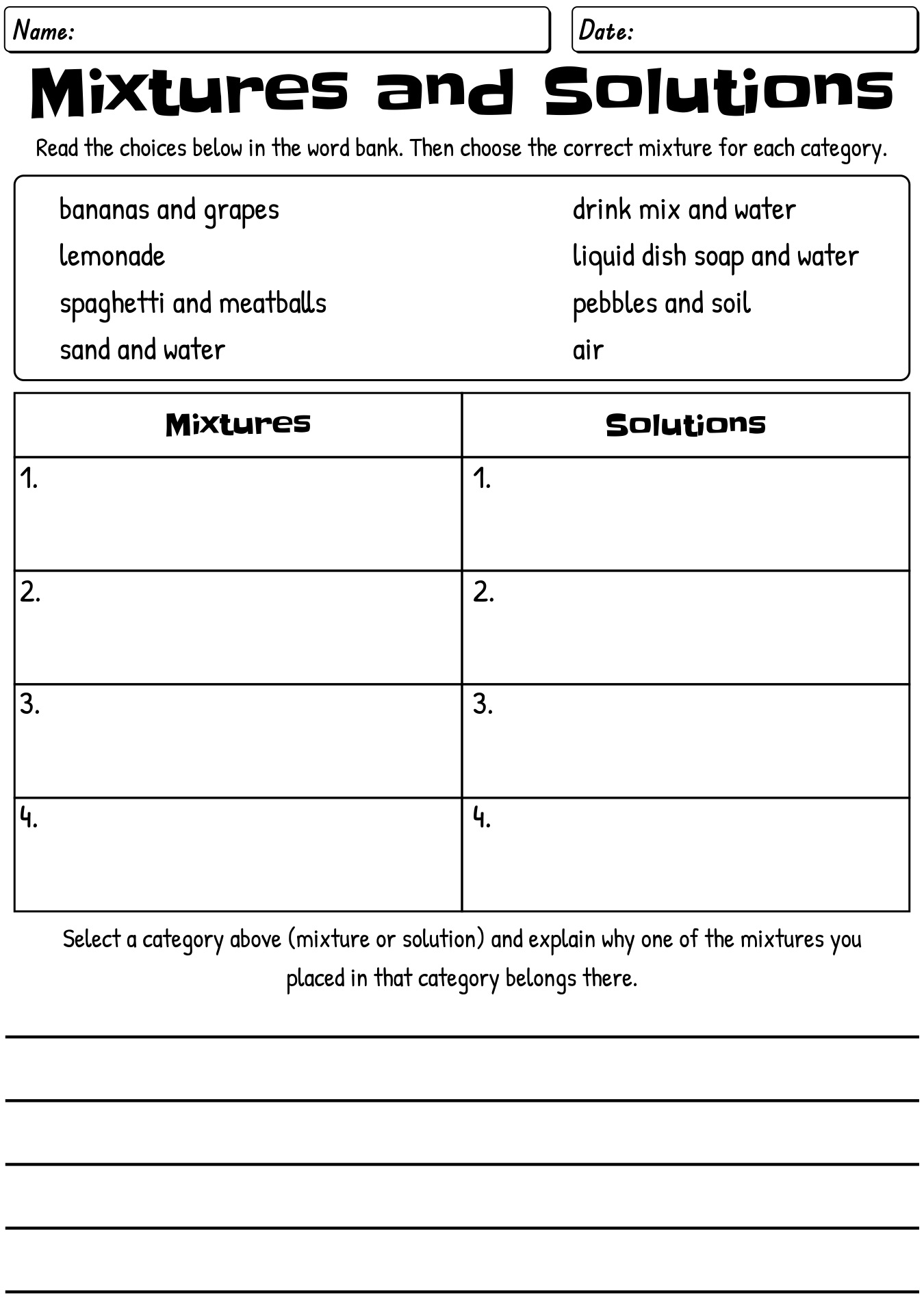
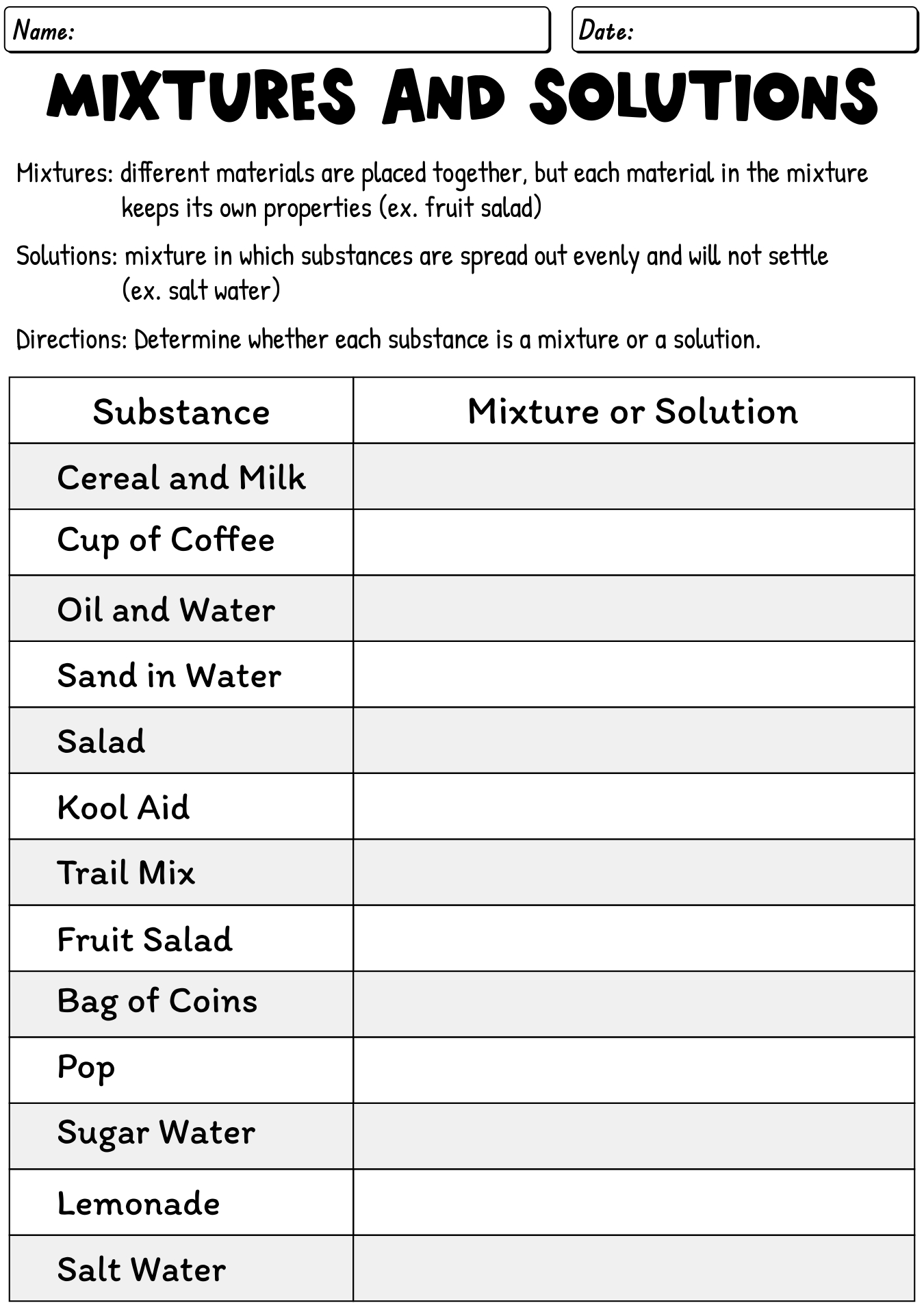
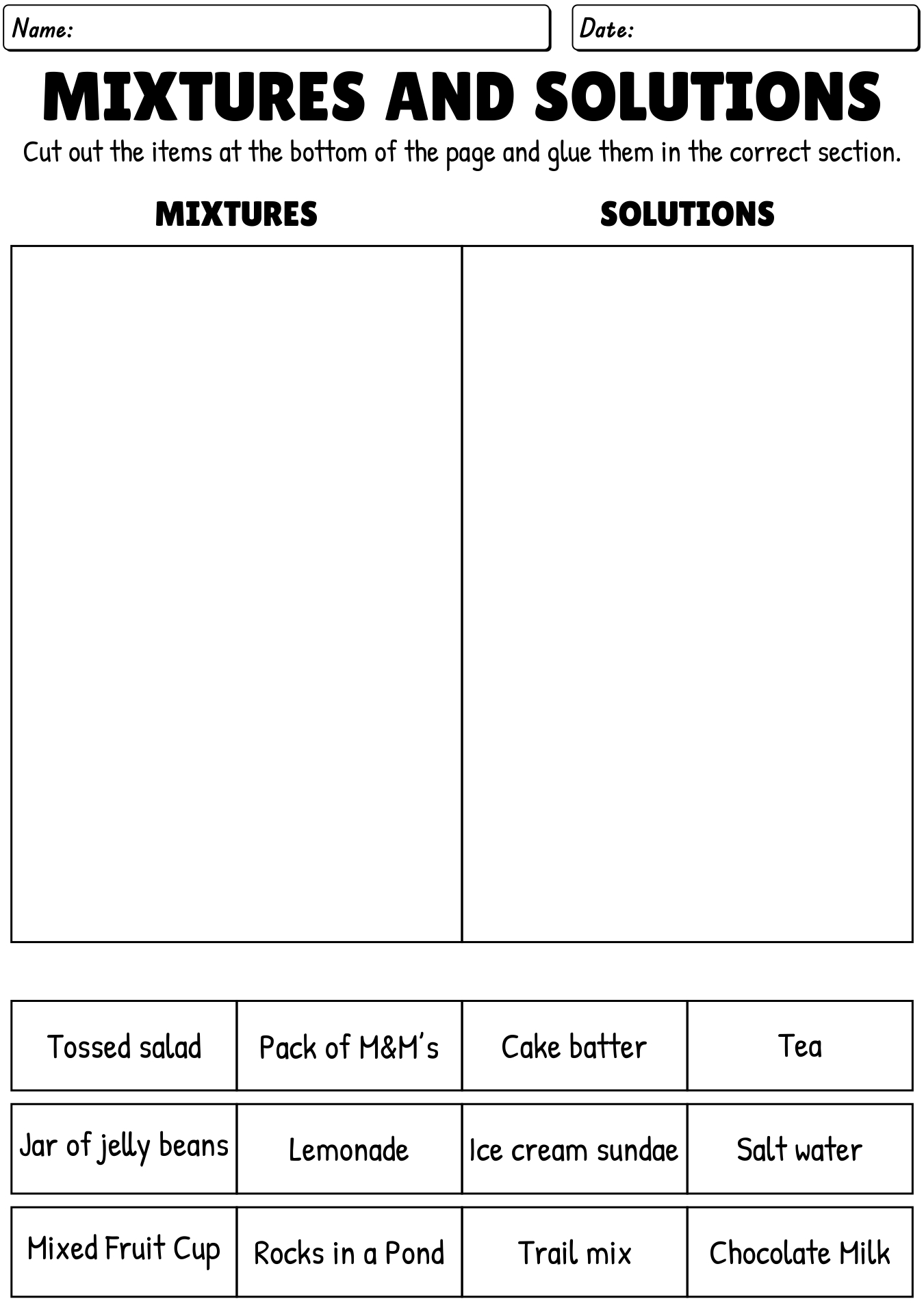
What is a mixture?
A mixture is a combination of two or more substances that are not chemically bonded together and can be separated by physical means, such as filtration or distillation. Each component in a mixture retains its own chemical properties and can exist in varying proportions.
Give an example of a homogeneous mixture.
An example of a homogeneous mixture is sugar dissolved in water to create a solution. In this mixture, the sugar molecules disperse evenly throughout the water, resulting in a uniform composition where the sugar cannot be visually distinguished from the water.
Describe the process of filtration.
Filtration is a physical process that involves the separation of solids from a liquid or gas mixture through a porous medium, such as a filter paper or membrane. The mixture is passed through the filter, allowing the liquid or gas to pass through while trapping the solid particles. Filtration is commonly used in various industries and laboratories to purify substances, remove impurities, and isolate particulate matter from a fluid. It is an effective method for separating different components based on their particle size and properties, providing a clean and clarified product.
What is solubility?
Solubility is the ability of a substance to dissolve in a solvent to form a homogeneous solution. It is typically measured as the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Solubility plays a crucial role in various chemical processes, including dissolution, precipitation, and crystallization.
Explain the difference between a solute and a solvent.
A solute is a substance that is dissolved in a solvent to create a solution, while a solvent is the substance that dissolves the solute. In a solution, the solute is typically present in smaller quantities compared to the solvent. The solute can be a solid, liquid, or gas, while the solvent is usually a liquid, but can also be a gas or a solid. The interaction between a solute and a solvent forms a homogenous mixture that is known as a solution.
What is a solution?
A solution is a homogeneous mixture composed of two or more substances, where one substance (the solute) is dissolved in another substance (the solvent). The solute can be solid, liquid, or gas, while the solvent is typically a liquid. Solutions are commonly found in various forms in everyday life, such as salt dissolved in water or sugar dissolved in coffee.
Give an example of a heterogeneous mixture.
Trail mix is an example of a heterogeneous mixture, as it is a combination of various ingredients such as nuts, dried fruits, chocolate chips, and pretzels that can be easily distinguished and separated from each other within the mixture.
Describe the process of distillation.
Distillation is a process of separating components of a liquid mixture based on differences in their boiling points. The mixture is heated to vaporize the components, and then the vapor is cooled and condensed back into liquid form, resulting in the separation of the components based on their different boiling points. The component with the lowest boiling point will vaporize first, while the component with the highest boiling point will remain in liquid form. This process allows for the purification and isolation of different components in a mixture.
What is concentration?
Concentration refers to the focus and effort directed towards a specific task or object. It involves devoting all mental and physical resources to a single activity, often leading to increased efficiency and productivity. This mental state allows individuals to block out distractions and maintain attention on the task at hand, leading to improved performance and outcomes.
Explain the concept of saturation in a solution.
Saturation in a solution refers to a state where the solution has reached its maximum capacity to dissolve a solute at a given temperature and pressure. At this point, any additional solute added will not dissolve and will instead settle at the bottom of the solution as a solid. Saturation is a key concept in chemistry and is often used to describe the equilibrium reached between the dissolved and undissolved components in a solution.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments