Writing Ionic Compounds Worksheet
Ionic compounds are a fundamental aspect of chemistry, and mastering their properties and formulas is crucial for anyone studying the subject. Whether you are a high school student preparing for an upcoming exam or a college student looking to refresh your knowledge, worksheets can be a valuable resource to help solidify your understanding of ionic compounds. By providing practice problems and opportunities for engagement, worksheets can aid in reinforcing key concepts and improving overall comprehension.
Table of Images 👆
- Writing Formulas Criss Cross Method Worksheet Answers
- Practice Naming Ionic Compounds Worksheet Answers
- Chemical Formula Writing Worksheet
- Naming Binary Ionic Compounds Worksheet Answers
- Naming Ionic Compounds Worksheet
- Binary Ionic Compounds Worksheet Answers
- Salt Chemical Formula
- Naming Ionic Compounds Worksheet Answer Key
- Writing Formulas Criss Cross Method Worksheet Answers
- Naming Ionic Compounds Worksheet Answers
- Chemistry Writing Chemical Equations Worksheet
- Chemistry Formula Sheet
- As Periodic Table of Elements
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is the purpose of the Writing Ionic Compounds Worksheet?
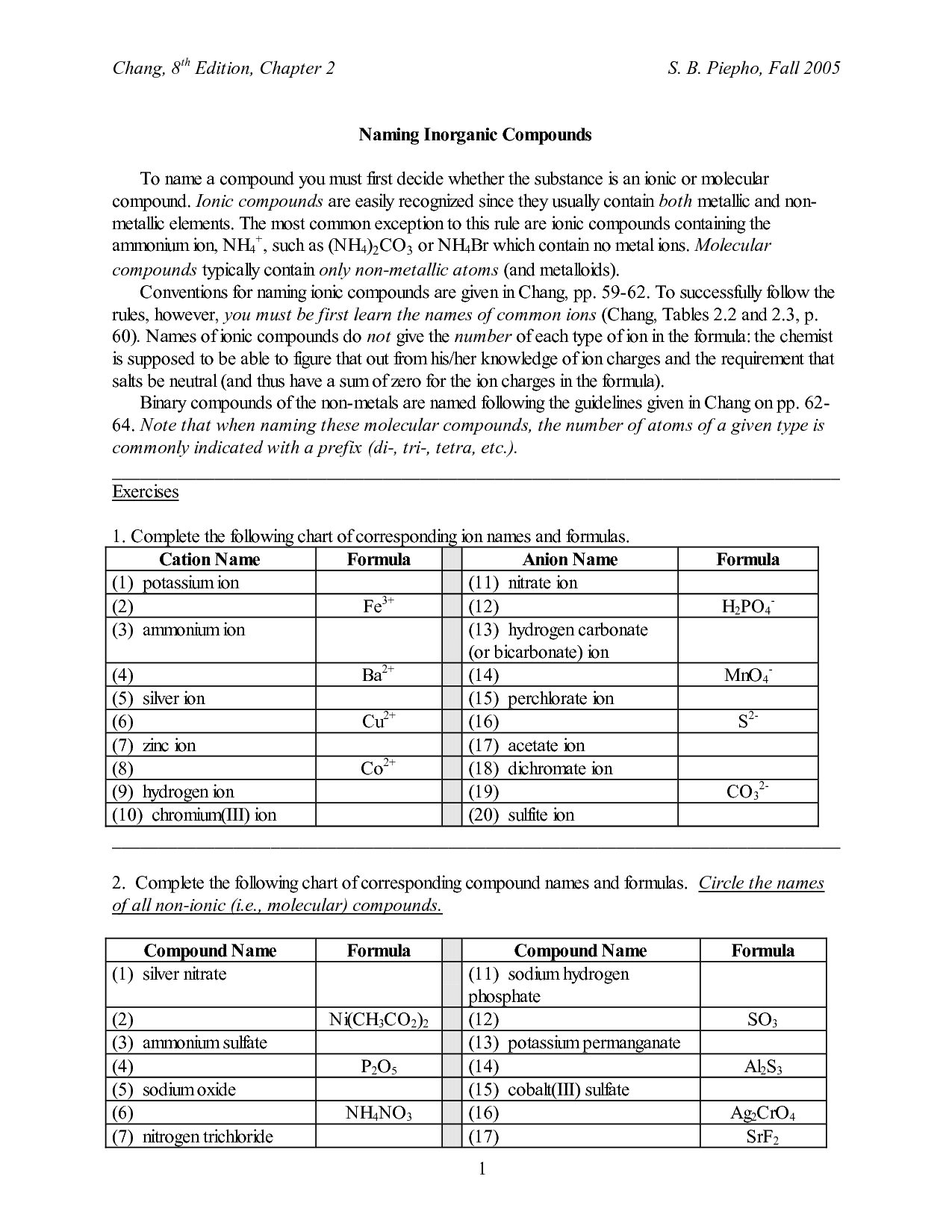
The purpose of the Writing Ionic Compounds Worksheet is to help students practice and reinforce their understanding of how ionic compounds are formed through the combination of positive and negative ions. By providing a structured format for writing the formulas of ionic compounds, the worksheet allows students to build their skills in identifying ions, determining their charges, and balancing them to create a neutral compound. This hands-on practice helps students grasp the concepts of ionic bonding and compound formation more effectively.
What are the key components of an ionic compound?
The key components of an ionic compound are positively charged ions, known as cations, and negatively charged ions, known as anions. These ions are held together by electrostatic forces of attraction due to the transfer of electrons from the cation to the anion during the formation of the compound. This results in a stable, neutral compound that has a lattice structure.
How do you determine the formula of an ionic compound?
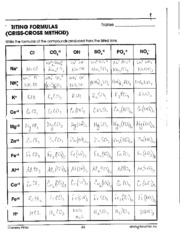
To determine the formula of an ionic compound, you need to identify the charges of the ions involved and balance them in a way that the overall charge is neutral. This is done by using the criss-cross method, where the absolute values of the charges are swapped between the ions to form subscripts in the formula. For example, in sodium chloride (NaCl), sodium has a +1 charge and chloride has a -1 charge, leading to the formula NaCl where the charges balance each other out.
What is the difference between a cation and an anion?
A cation is a positively charged ion that forms when an atom loses one or more electrons, while an anion is a negatively charged ion that forms when an atom gains one or more electrons. Cations have a net positive charge due to having more protons than electrons, while anions have a net negative charge due to having more electrons than protons.
How do you identify the charges of ions in an ionic compound?
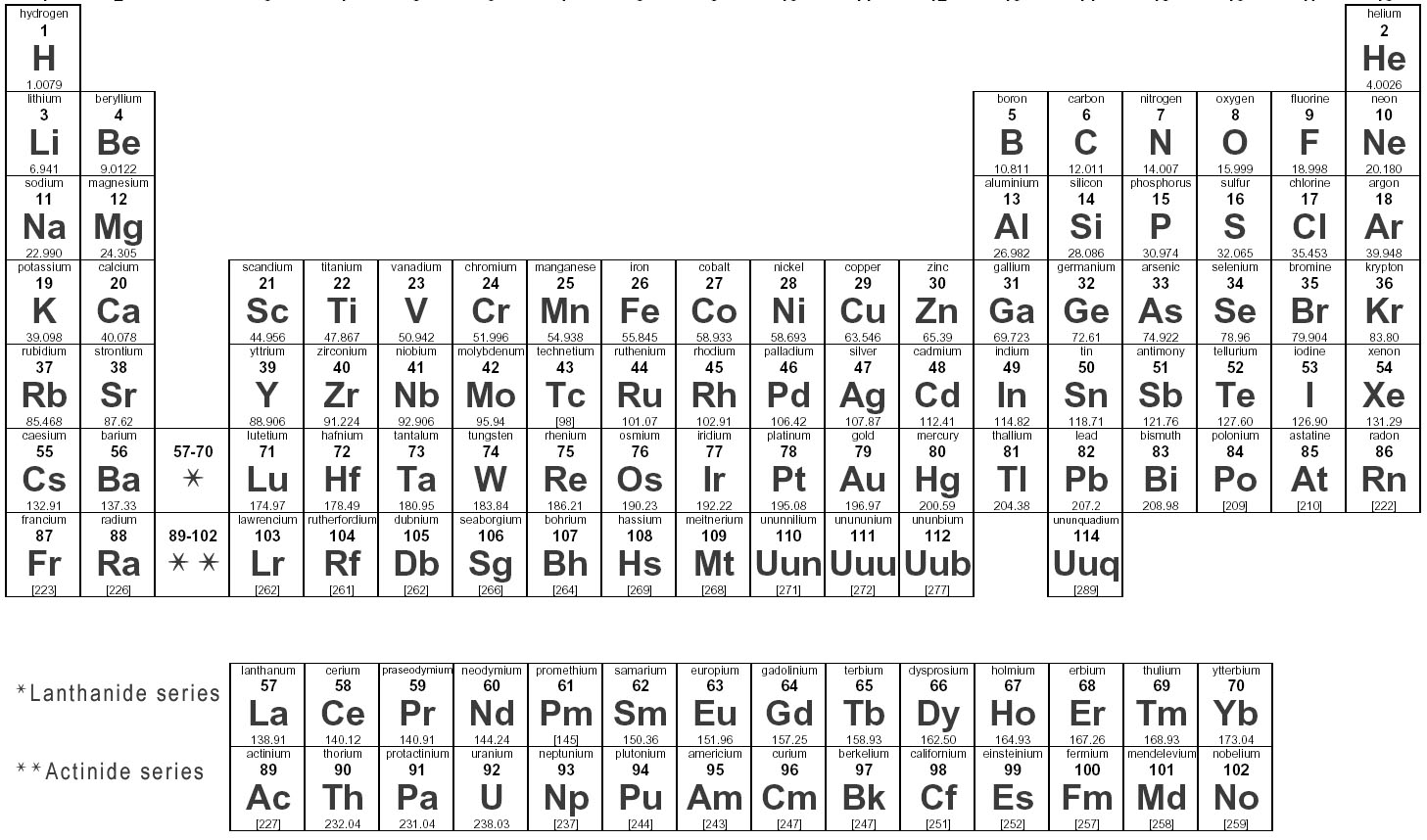
To identify the charges of ions in an ionic compound, you can refer to the periodic table to determine the typical charges of elements based on their group number. For example, elements in group 1 typically have a +1 charge, elements in group 2 have a +2 charge, and elements in group 17 have a -1 charge. Once you know the charges of the individual ions, you can balance them in the compound to ensure that the overall charge is neutral.
What are some examples of common ionic compounds?
Some examples of common ionic compounds include sodium chloride (table salt, NaCl), calcium carbonate (limestone, CaCO3), potassium iodide (KI), magnesium sulfate (Epsom salt, MgSO4), and ammonium nitrate (NH4NO3).
How do you balance charges when writing the formula of an ionic compound?
To balance charges when writing the formula of an ionic compound, you need to ensure that the overall positive charge from the cations is equal to the overall negative charge from the anions. This is achieved by using subscripts to indicate the number of cations and anions needed to balance the charges. The subscripts should be the smallest whole numbers possible to maintain charge neutrality in the compound. Remember that the charges of the cations and anions must be taken into account when balancing charges in the formula of an ionic compound.
What are some guidelines for naming ionic compounds?
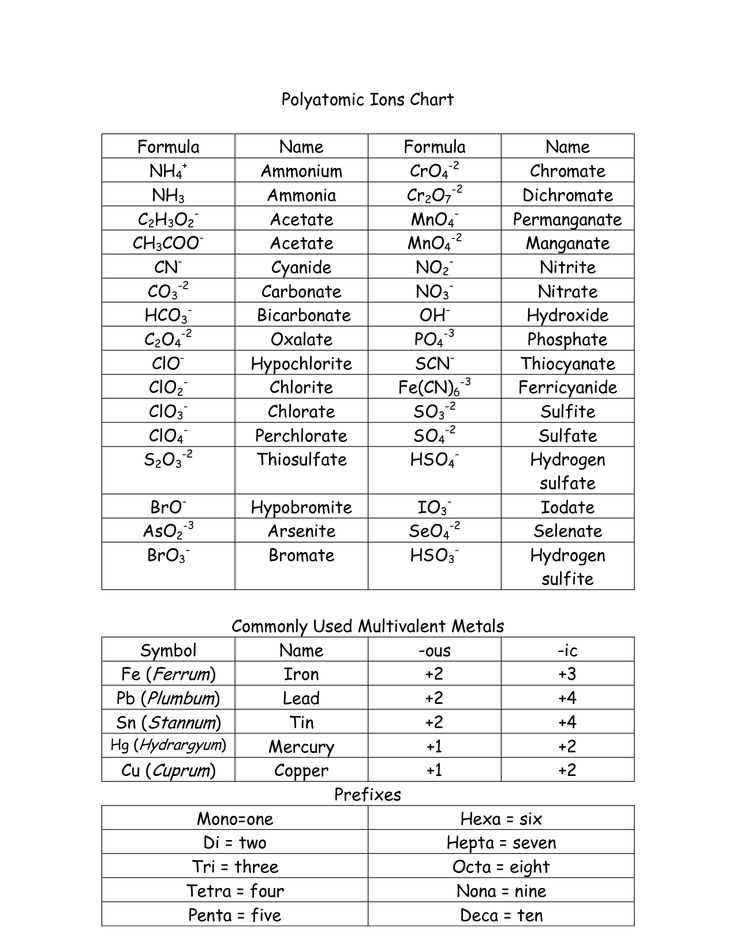
When naming ionic compounds, the cation (positively charged ion) is named first followed by the anion (negatively charged ion). The name of the cation is typically just the name of the element, while for the anion, the ending of the element's name is replaced with "-ide." Roman numerals are used to indicate the charge of the cation when dealing with transition metals that can have multiple oxidation states. Additionally, some common polyatomic ions have specific names that should be memorized.
How are polyatomic ions involved in the writing of ionic compounds?
Polyatomic ions are groups of atoms that carry a charge and act as a single unit in chemical reactions. In the writing of ionic compounds, polyatomic ions are considered as single entities when determining the overall charge balance of the compound. They are treated as a single unit and can bond with other ions to form a stable compound through ionic bonding. Polyatomic ions play a crucial role in creating a balanced equation for ionic compounds by allowing for the transfer of electrons between the ions to achieve an overall neutral charge in the compound.
What are some strategies for checking the correctness of your written ionic compound formulas?
There are a few strategies to check the correctness of your written ionic compound formulas. First, make sure the charges of the ions balance out to zero in the compound. You can use the crisscross method to determine the correct subscripts for each ion. Additionally, verify that the sum of the positive charges from cations equals the sum of the negative charges from anions in the compound. Finally, reference a periodic table to confirm the charges of the ions you are working with match the expected values for each element.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments