Worksheets Naming Carboxylic Acids
Worksheet activities are incredibly helpful when it comes to reinforcing learning and providing additional practice on specific topics. For those students or educators studying carboxylic acids, these worksheets provide a structured and engaging way to delve into the naming of carboxylic acids.
Table of Images 👆
- Carboxylic Acid Worksheet
- Carboxylic Acid Group Structure
- Mutations Worksheet Answer Key
- Organic Chemistry Nomenclature Worksheet
- Naming Organic Compounds Worksheet
- Alkane Alkene Alkyne Naming Worksheet with Answers
- Organic Functional Groups Worksheet
- Organic Chemistry Nomenclature Worksheets with Answers
- Covalent Bonding Study Guide Answers
- Isomers of Hexane Structural Formula
- Organic Chemistry Naming Alkanes Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What are carboxylic acids?
Carboxylic acids are organic compounds characterized by a carboxyl functional group, which consists of a carbon atom double-bonded to an oxygen atom and bonded to a hydroxyl group (?COOH). They are acidic in nature due to the release of a proton from the hydroxyl group when dissolved in water. Carboxylic acids are commonly found in nature and play vital roles in various biological processes, as well as in many industrial applications such as food preservation and pharmaceuticals.
How are carboxylic acids formed?
Carboxylic acids are formed through the oxidation of primary alcohols or aldehydes, where the carbonyl group is converted into a carboxyl group (–COOH) with the loss of water. This oxidation can be achieved through various methods, such as using oxidizing agents like potassium permanganate or chromic acid. Additionally, carboxylic acids can also be produced by the hydrolysis of nitriles or esters.
What is the general formula of carboxylic acids?
The general formula of carboxylic acids is RCOOH, where R represents an alkyl group or hydrogen. This functional group consists of a carbonyl group (C=O) bonded to a hydroxyl group (–OH) on the same carbon atom, giving carboxylic acids their characteristic acidity and reactivity.
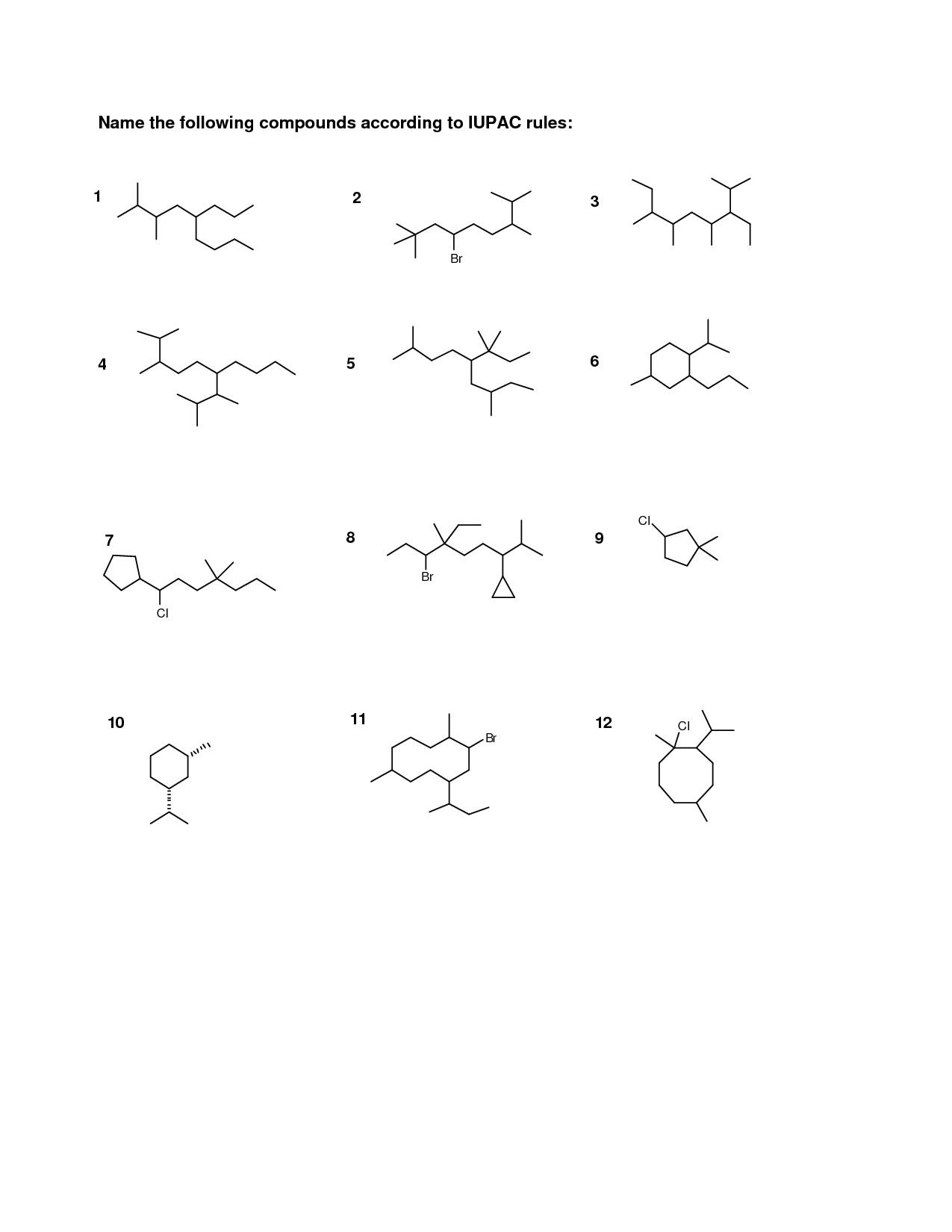
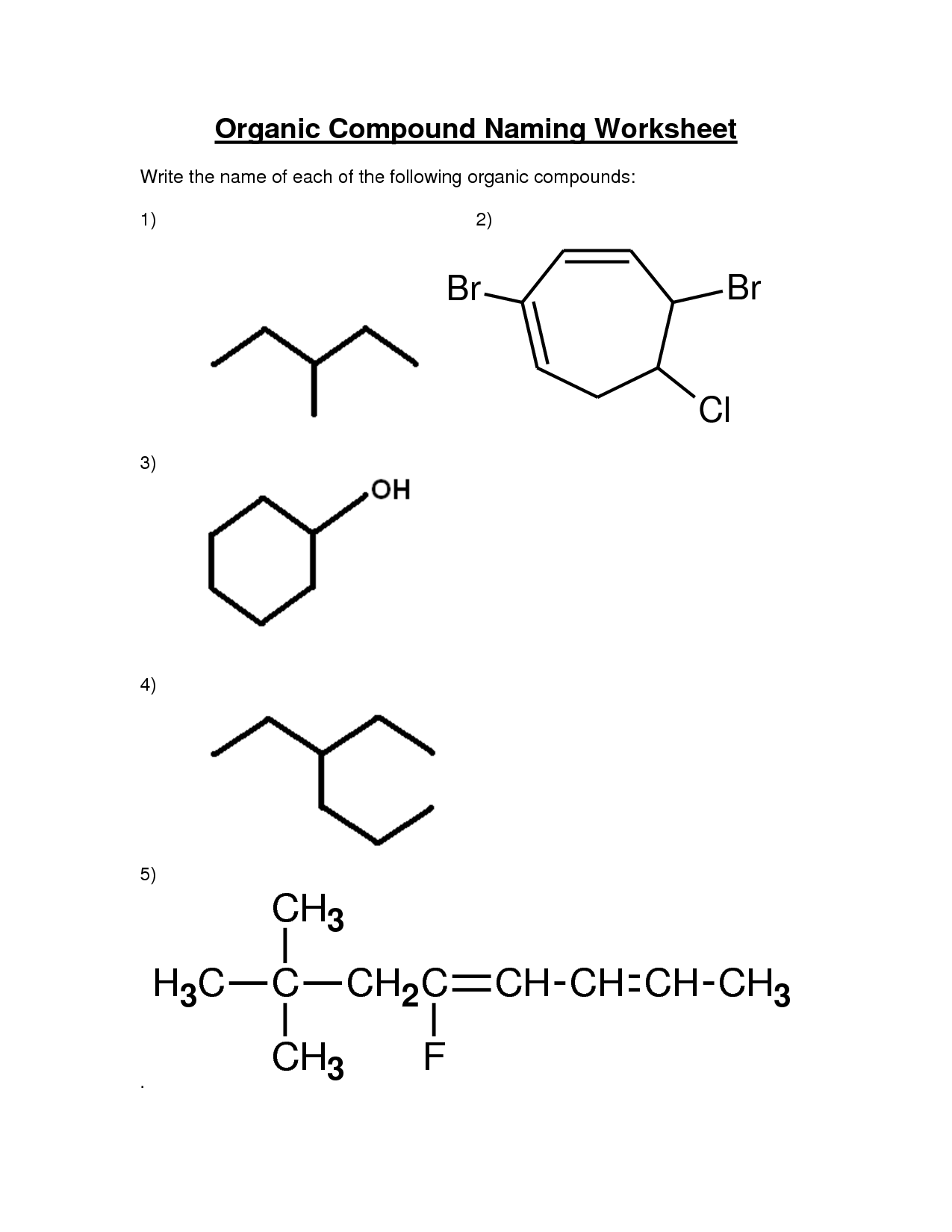
How do you name a carboxylic acid?
To name a carboxylic acid, you use the suffix "-oic acid" in the name of the parent alkane or alkene chain. The carboxylic acid functional group is always located at the end of the chain, and you number the carbons in the chain to give the carboxylic acid the lowest possible number. If there are substituents, you name and number them accordingly. For example, if you have a carboxylic acid with a three-carbon chain, it would be named propanoic acid.
How do you indicate the position of substituents in a carboxylic acid?
The position of substituents in a carboxylic acid is indicated by assigning numbers to the carbon atoms in the carboxyl group. The carbon atom of the carboxyl group directly bonded to the carboxyl oxygen is considered carbon 1, and subsequent carbon atoms are numbered accordingly. Substituents are then located by stating the carbon number to which they are attached in the chemical name, indicated by the prefix before the parent carboxylic acid name.
What are common naming prefixes for carboxylic acids?
Common naming prefixes for carboxylic acids include form-, acet-, propion-, butyr-, valer-, capro-, and enantio- depending on the number of carbon atoms in the carbon chain of the acid.
How do you name carboxylic acids with multiple substituents?
To name carboxylic acids with multiple substituents, you need to identify the parent chain containing the carboxylic acid group, and then list the substituents alphabetically. The carboxylic acid group is always given the highest priority, making it the main functional group. The position of each substituent is indicated by a number, starting from the carboxylic acid carbon as carbon 1. If there are multiple identical substituents, use prefixes like di-, tri-, tetra-, etc. Lastly, the names of the substituents are hyphenated and written before the parent chain name.
What are the rules for naming cyclic carboxylic acids?
To name cyclic carboxylic acids, start by identifying the parent carbon chain with the carboxylic acid group. Number the carbon atoms in the ring to give the carboxylic acid group the lowest possible locant number. Then, add the suffix "-oic acid" to indicate the carboxylic acid functional group. If the carboxylic acid is a substituent on a larger carbon chain, name it as a substituent by adding the prefix "carboxyl-" followed by the name of the parent alkane chain.
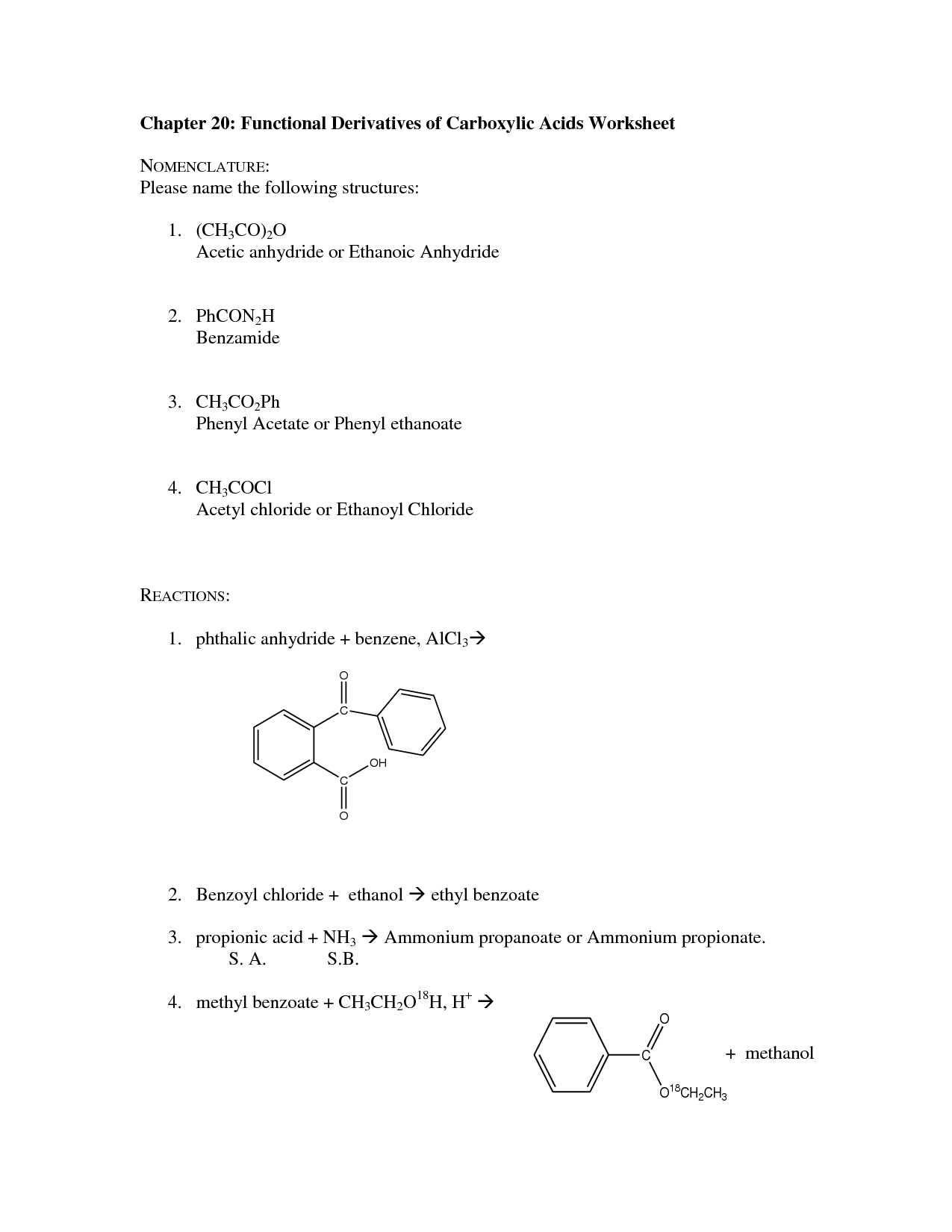
How do you name carboxylic acid derivatives?
Carboxylic acid derivatives are named by first identifying the parent carboxylic acid compound, and then specifying the functional group on the derivative. For example, esters are named by replacing the "-ic acid" ending of the parent carboxylic acid with "-ate", while amides replace it with "-amide". For anhydrides, the word "anhydride" is added at the end of the parent carboxylic acid name. It's important to know the specific suffixes for each type of carboxylic acid derivative to properly name them.
How do you name complex carboxylic acids with multiple functional groups?
To name complex carboxylic acids with multiple functional groups, first identify and prioritize the carboxylic acid group. Then, locate and name the other functional groups using appropriate prefixes indicating their positions. Number the carbon chain with the carboxylic acid group as carbon 1, and assign locants to the other functional groups based on their positions. Finally, list the functional groups in alphabetical order with their locants and the carboxylic acid at the end, using hyphens to separate numbers and commas to separate numbers from letters.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments