Water Molecule Worksheet
Are you a student studying chemistry or a science enthusiast interested in learning more about the properties of water molecules? If so, you've come to the right place! In this blog post, we will introduce you to a water molecule worksheet that will help you further your understanding of this fundamental entity and its unique characteristics.
Table of Images 👆
- Photosynthesis and Cellular Respiration
- Virtual Lab Population Biology Worksheet Answers
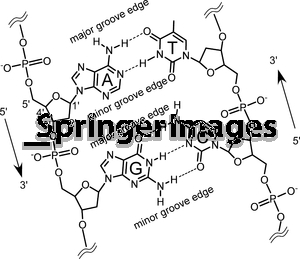
- Deoxyribose DNA Structure
- Life Molecules Worksheet
- Bill Nye Atmosphere Worksheet
- BrainPOP pH Scale Worksheet Answer Key
- Polar and Nonpolar Functional Groups
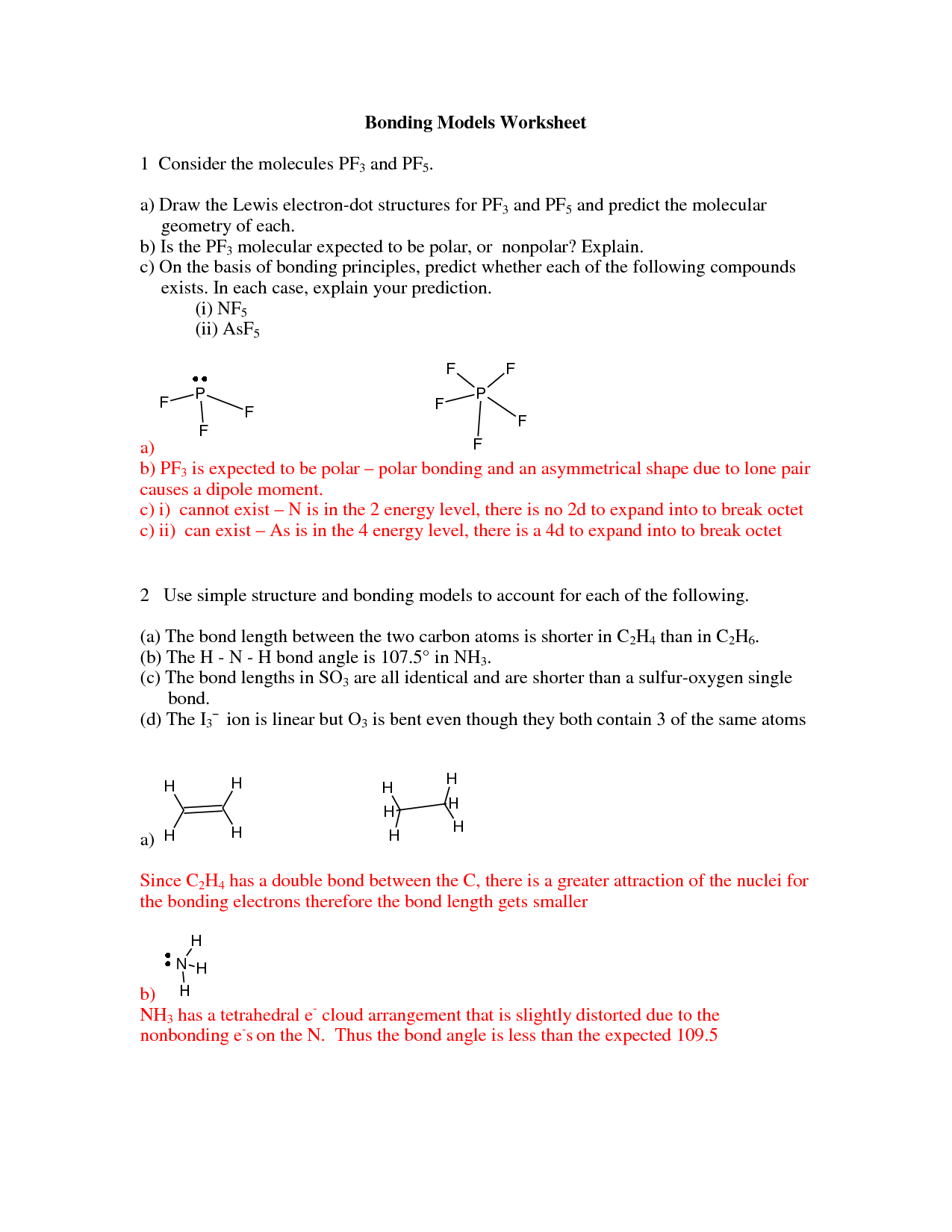
- C2H4 Lewis Structure Molecular Geometry
- Acid-Base Balance Summary during Exercise
- Modern Biology Chapter 8 Notes
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the chemical formula of a water molecule?
The chemical formula of a water molecule is H2O, which represents two hydrogen atoms bonded to one oxygen atom.
How many atoms are present in a water molecule?
A water molecule consists of three atoms - two hydrogen atoms and one oxygen atom, H2O.
What type of bond holds the hydrogen and oxygen atoms together in a water molecule?
A polar covalent bond holds the hydrogen and oxygen atoms together in a water molecule. This type of bond forms when electrons are shared between the atoms unequally, resulting in a slightly positive charge on the hydrogen atoms and a slightly negative charge on the oxygen atom.
Describe the shape of a water molecule.
A water molecule has a bent or V-shaped structure due to the arrangement of its two hydrogen atoms and one oxygen atom. The oxygen atom is located at the center with two hydrogen atoms bonded at an angle of approximately 104.5 degrees to it, creating a distinctive shape.
What is the overall charge of a water molecule?
The overall charge of a water molecule is neutral, as it consists of two positively charged hydrogen atoms and one negatively charged oxygen atom, resulting in a balanced electrical charge.
What property of water molecules makes them capable of forming hydrogen bonds with each other?
The property of water molecules that allows them to form hydrogen bonds with each other is their polarity. Water molecules have a slightly negative oxygen atom and two slightly positive hydrogen atoms, creating a dipole moment. This polarity allows the oxygen atom of one water molecule to attract the hydrogen atoms of neighboring water molecules, creating hydrogen bonds that contribute to the unique properties of water, such as high surface tension, cohesion, and the ability to moderate temperature changes.
Explain why water molecules are considered polar.
Water molecules are considered polar because they have a asymmetrical distribution of charge. This is due to the unequal sharing of electrons between the oxygen and hydrogen atoms in the molecule. Oxygen has a higher electronegativity compared to hydrogen, leading to a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. As a result, water molecules exhibit a positive and negative end, making them polar molecules.
How does the polarity of water molecules contribute to its unique properties, such as high boiling point and surface tension?
The polarity of water molecules, with oxygen being more electronegative than hydrogen, results in a slight negative charge near the oxygen atom and a slight positive charge near the hydrogen atoms. This polarity allows water molecules to form hydrogen bonds with each other, leading to cohesion, high boiling point, and surface tension. Hydrogen bonding causes water molecules to stick together strongly, requiring more energy to separate them (high boiling point) and creating a "skin" on the surface (surface tension). These unique properties make water essential for life and various natural phenomena.
Describe the process of hydrogen bonding between water molecules.
Hydrogen bonding in water molecules occurs when the positively charged hydrogen atom of one water molecule is attracted to the negatively charged oxygen atom of another water molecule. This attraction forms a weak bond, creating a network of interconnected water molecules. The hydrogen bonding in water gives it unique properties such as high surface tension, cohesion, and the ability to absorb and release heat effectively, making it essential for life on Earth.
How does the structure of a water molecule affect its ability to dissolve a wide range of substances?
The structure of a water molecule, comprising one oxygen atom and two hydrogen atoms, gives it a polar nature with slight positive and negative charges. This polarity allows water to form hydrogen bonds with other molecules, enabling it to break apart and surround solute particles, eventually dissolving them. Additionally, the shape of the water molecule gives it a high level of cohesion and adhesion, helping it to interact effectively with various substances and facilitate the dissolution process.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments