Types of Reactions Worksheet Answers
Are you a chemistry student looking for a reliable resource to practice different types of reactions? Look no further, because in this blog post, we will be providing you with the answers to a Types of Reactions Worksheet. This resource is designed for anyone studying chemistry and wanting to solidify their understanding of the various types of chemical reactions.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answer Key
- Chemical Reaction Types Worksheet
- Identifying Chemical Reaction Types Worksheet
- Types of Chemical Reactions Worksheet Answer Key
- Chemical Reactions Predicting Products Worksheet
- Spinal Cord Reflex Arc Diagram
- Balancing Chemical Equations Worksheet Answer Key
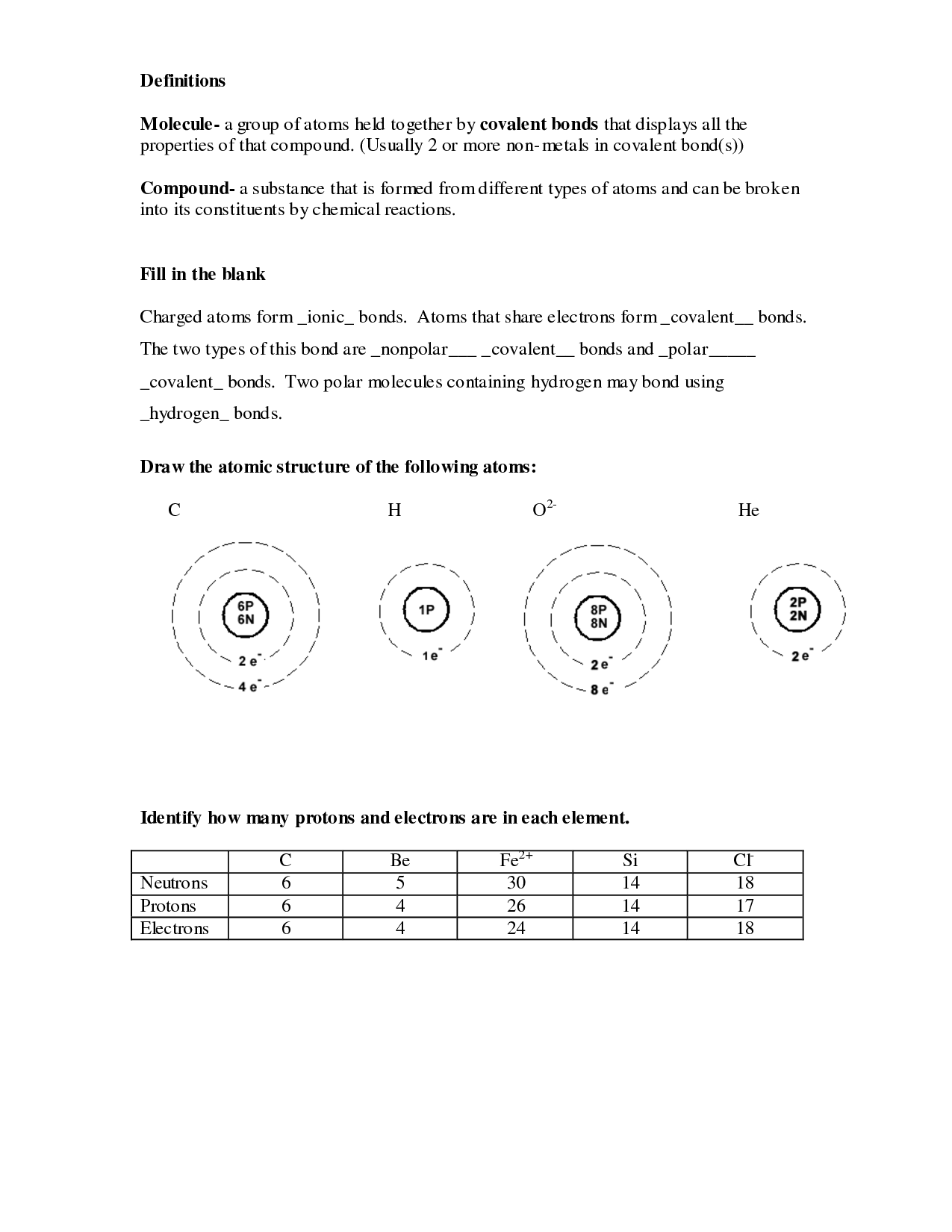
- Atomic Structure Worksheet Answers
- Mole Molecules and Grams Worksheet Answer Key
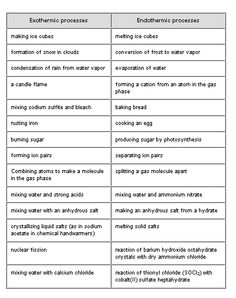
- Exothermic vs Endothermic Reactions Worksheet
- Balancing Chemical Equations Worksheet 1
- Double Replacement Reaction Examples
- Macromolecule Worksheet Answer Key
- Macromolecule Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
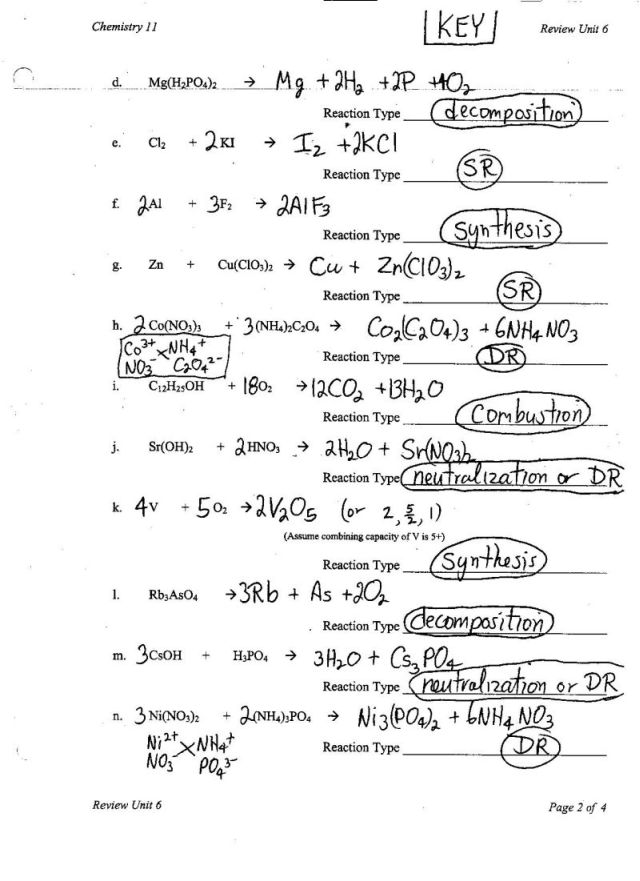
What is a synthesis reaction?
A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. This process typically involves the creation of a single compound from simpler reactants, often with the release of energy. Synthesis reactions are commonly used in organic chemistry to build more complex molecules from simpler building blocks.
A synthesis reaction is a type of chemical reaction where two or more substances combine to form a more complex substance.
Correct, in a synthesis reaction, two or more simpler substances react to form a more complex compound. This type of chemical reaction typically involves the addition of elements or functional groups to form a new product.
What is a decomposition reaction?
A decomposition reaction is a type of chemical reaction in which a compound breaks down into simpler substances. This process is usually accomplished through the input of energy, whether in the form of heat, light, or electrical current, leading to the separation of the original compound into its constituent elements or other compounds.
A decomposition reaction is a type of chemical reaction where a single compound breaks down into two or more simpler substances.
Yes, a decomposition reaction is a type of chemical reaction in which a single compound is broken down into two or more simpler substances.
What is a combustion reaction?
A combustion reaction is a type of chemical reaction in which a substance reacts rapidly with oxygen, often producing heat and light as energy is released. This reaction typically involves a hydrocarbon or another fuel source combining with oxygen to form carbon dioxide and water, along with releasing energy in the form of heat and light.
A combustion reaction is a type of chemical reaction that involves the rapid combination of a substance with oxygen, resulting in the release of heat, light, and often a flame.
Yes, a combustion reaction is a type of chemical reaction that involves the rapid combination of a substance with oxygen, resulting in the release of heat, light, and often a flame.
What is a single replacement reaction?
A single replacement reaction is a type of chemical reaction where an element replaces another element in a compound, typically with the more reactive element displacing the less reactive one. This results in the formation of a new compound and a different element.
A single replacement reaction is a type of chemical reaction where one element displaces another element in a compound, resulting in the formation of a new compound and a different element.
Yes, that is correct. In a single replacement reaction, one element switches places with another element in a compound, producing a new compound and releasing a different element in the process. This type of reaction is also known as a displacement reaction due to the replacement of one element by another.
What is a double replacement reaction?
A double replacement reaction is a type of chemical reaction that occurs when the ions of two compounds exchange places, forming two new compounds. This reaction typically takes place in an aqueous solution and involves the ions in the compounds reacting with each other to form new ionic compounds.
A double replacement reaction is a type of chemical reaction where the positive ion of one compound exchanges places with the positive ion of another compound, resulting in the formation of two new compounds.
Yes, that's correct! In a double replacement reaction, the cations and anions of two ionic compounds switch partners to form two new compounds. This type of reaction typically occurs in aqueous solutions and can be identified by the formation of a precipitate or a gas.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments