Types of Chemical Reactions Worksheet Answers
Chemistry students, who find themselves in need of practice and reinforcement on various types of chemical reactions, can access worksheets specifically designed for this purpose. These worksheets provide answers, enabling students to assess their grasp on the subject matter and achieve a deeper understanding of different chemical reactions.
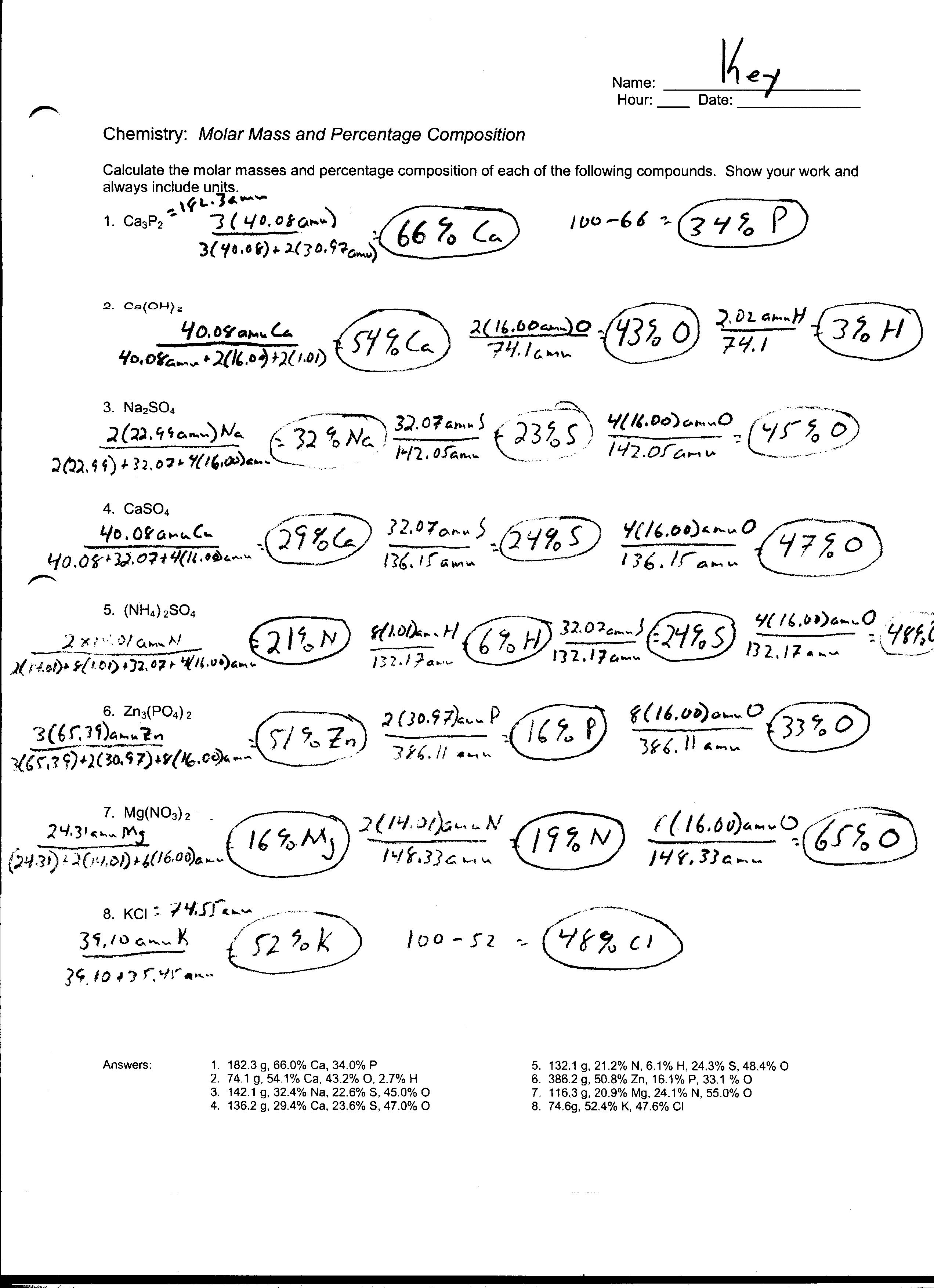
Table of Images 👆
- Virtual Lab Enzyme-Controlled Reactions Answer Key
- Double Replacement Reaction Worksheet Answers
- Predicting Reaction Products Worksheet Answers
- Balancing Equations Worksheet Answer Key
- Chemistry Word Equations Worksheet Answer Key
- Mass to Mole Stoichiometry Worksheet Answer Key
- Balancing Chemical Equations Worksheet Answer Key
- Modern Biology Study Guide Answer Key
- Balancing Chemical Equations Worksheet 1
- Chemistry Stoichiometry Worksheet Answer Key
- Molarity Practice Problems Worksheet Answers
- Molarity Practice Problems Worksheet Answers
- Molarity Practice Problems Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is a synthesis reaction?
A synthesis reaction, also known as a combination reaction, is a chemical reaction where two or more substances combine to form a single, more complex product. This type of reaction typically involves the rearrangement and bonding of atoms to create a new compound.
What is a decomposition reaction?
A decomposition reaction is a type of chemical reaction in which a compound breaks down into simpler substances or elements. This process is usually triggered by heat, electricity, or another chemical reaction. During a decomposition reaction, the bonds holding the compound together are broken, resulting in the formation of new substances.
What is a combustion reaction?
A combustion reaction is a type of chemical reaction that occurs when a substance reacts rapidly with oxygen to produce heat and typically light in the form of a flame. This reaction involves the combustion of a fuel, such as a hydrocarbon, in the presence of oxygen to produce carbon dioxide, water, and heat energy as products.
What is a single displacement reaction?
A single displacement reaction is a type of chemical reaction where an element displaces another element in a compound, resulting in the formation of a new compound and a separate elemental substance. This reaction occurs when a more reactive element displaces a less reactive element from its compound.
What is a double displacement reaction?
A double displacement reaction is a type of chemical reaction in which two compounds react by exchanging ions to form two new compounds. This reaction typically occurs in aqueous solutions and involves the formation of a precipitate, gas, or water as a product.
What is a redox reaction?
A redox (reduction-oxidation) reaction is a chemical reaction in which one substance loses electrons (oxidation) while another substance gains electrons (reduction). This transfer of electrons results in a change in the oxidation states of the reactants. Redox reactions are essential in many biological processes, industrial applications, and everyday chemical reactions.
What is an acid-base reaction?
An acid-base reaction is a chemical process in which an acid and a base react with each other to form a salt and water. The reaction involves the transfer of protons (H+ ions) between the acid and the base, resulting in the formation of an ionic compound (salt) and the neutralization of the acidic and basic properties. This type of reaction is important in many biological, industrial, and environmental processes.
What is a precipitation reaction?
A precipitation reaction is a chemical reaction in which soluble reactants combine to form an insoluble solid compound, known as a precipitate. This occurs when the product of the reaction is insoluble in the solvent, causing it to separate out from the solution and appear as a solid.
What is a neutralization reaction?
A neutralization reaction is a chemical reaction in which an acid and a base react to form water and a salt. In this reaction, the acidic properties of the acid and the basic properties of the base are neutralized, resulting in the formation of water and a salt as the products.
What is a displacement reaction?
A displacement reaction is a type of chemical reaction where a more reactive element displaces a less reactive element from its compound. This results in the formation of a new compound with the more reactive element and the original less reactive element being set free.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments