Specific Heat Capacity Worksheet Answers
If you're a student or teacher looking for a reliable resource to help reinforce your understanding of specific heat capacity, you've come to the right place. This blog post will provide you with the answers to a specific heat capacity worksheet, ensuring you have a solid grasp on this important physics concept. By reviewing the solutions provided, you can check your work, identify any areas for improvement, and build confidence in your knowledge of specific heat capacity.
Table of Images 👆
- Stoichiometry Worksheet Answer Key
- Calorimetry Worksheet Answers
- Specific Heat Capacity Formula
- Hesss Law Worksheet Answers
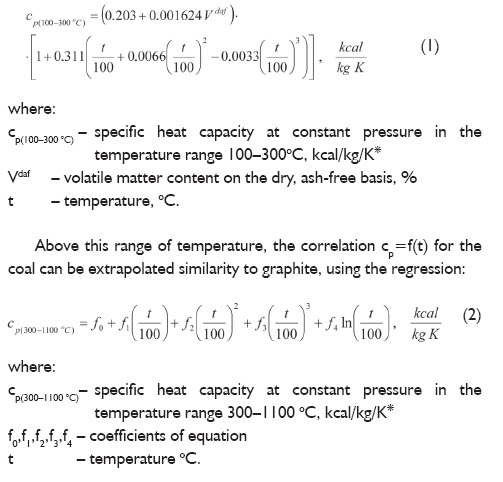
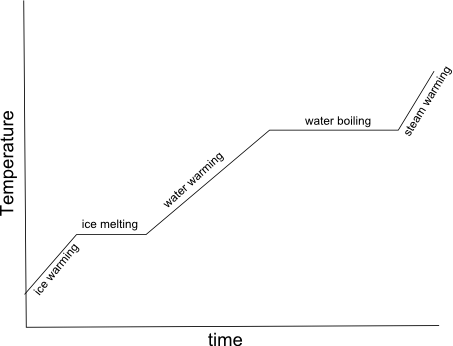
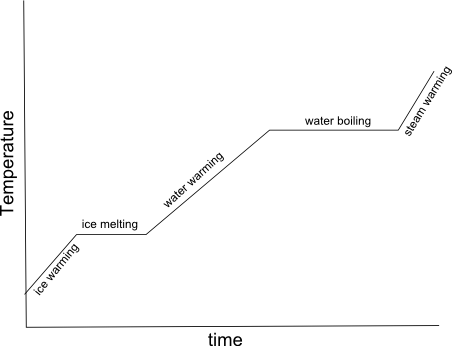
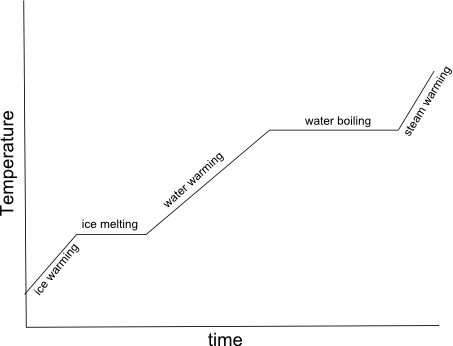
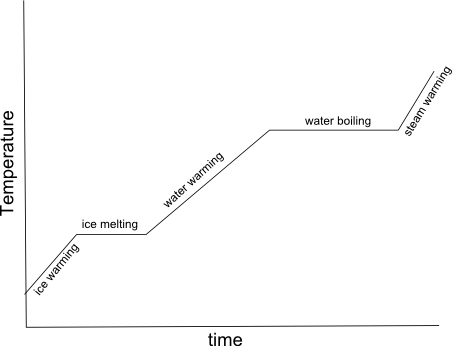
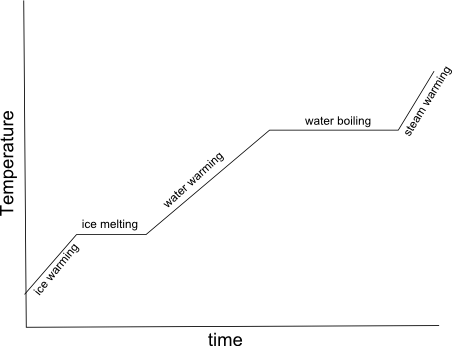
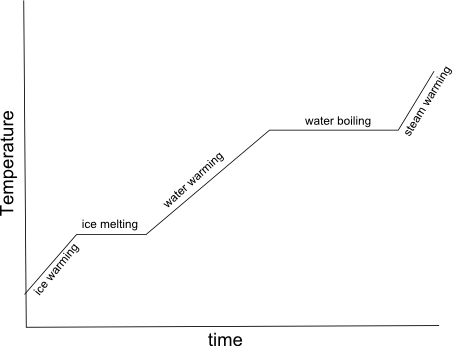
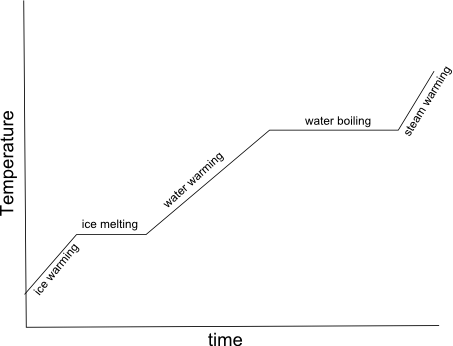
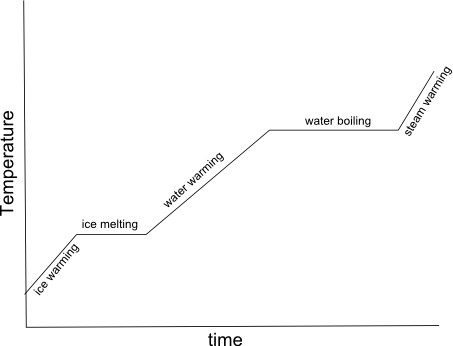
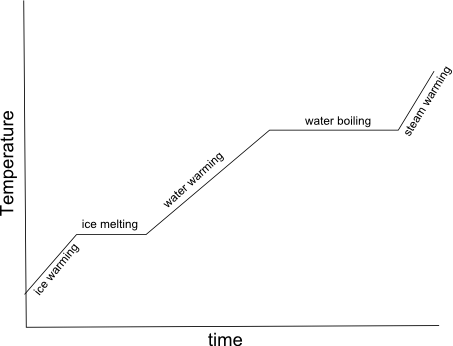
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
- Heating Curve Graph
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is specific heat capacity?
Specific heat capacity is the amount of heat energy required to raise the temperature of a unit mass (usually 1 gram or 1 kilogram) of a substance by 1 degree Celsius (or 1 Kelvin). It is an intrinsic property of a material and is often used to characterize and compare different substances in terms of how effectively they can absorb and store heat.
Specific heat capacity is the amount of heat energy required to raise the temperature of a substance by a certain amount.
Correct. The specific heat capacity of a substance is the amount of heat energy needed to raise the temperature of one unit mass of the substance by one degree Celsius or Kelvin. It is a fundamental property that varies from one material to another.
How is specific heat capacity different from heat capacity?
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius, while heat capacity is the amount of heat required to raise the temperature of an object by one degree Celsius. Specific heat capacity is an intensive property that is unique to a material, whereas heat capacity is an extensive property that depends on both the material and the amount of that material present.
Specific heat capacity is the amount of heat energy needed to raise the temperature of a unit mass of a substance, while heat capacity is the amount of heat energy required to raise the temperature of an entire object.
Specific heat capacity is the heat energy needed to raise the temperature of a unit mass of a substance, whereas heat capacity is the heat energy required to raise the temperature of an entire object.
Why is water often used as a reference substance for specific heat capacity calculations?
Water is often used as a reference substance for specific heat capacity calculations because it has a relatively high specific heat capacity compared to many other substances, meaning it can absorb and release large amounts of heat energy without significant changes in temperature. This makes water a useful benchmark for comparing the specific heat capacities of other substances and for understanding how different materials respond to changes in temperature. Additionally, water is a common and easily accessible substance, making it convenient for experimental measurements and calculations.
Water's specific heat capacity is relatively high and consistent, making it a convenient substance for comparisons and calculations.
Water's high and consistent specific heat capacity, which is the amount of heat energy required to raise its temperature, makes it a convenient substance for comparisons and calculations. This property allows water to absorb and release large amounts of heat without changing its temperature significantly, making it an important factor in regulating Earth's climate and sustaining life through temperature moderation in ocean and land ecosystems.
What is the formula for specific heat capacity?
The formula for specific heat capacity is given by \( Q = mc\Delta T \), where Q represents the amount of heat energy transferred, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
The formula for specific heat capacity is Q = mcΔT, where Q represents heat energy, m represents mass, c represents specific heat capacity, and ΔT represents the change in temperature.
Correct, the formula for specific heat capacity is Q = mcΔT, where Q represents heat energy, m represents mass, c represents specific heat capacity, and ΔT represents the change in temperature.
How is specific heat capacity measured?
Specific heat capacity is measured by applying a known amount of heat to a substance and measuring the resulting temperature change. This is typically done by using a calorimeter, which is a device designed to accurately measure heat exchange. By knowing the amount of heat added, the initial and final temperatures, and the mass of the substance, the specific heat capacity can be calculated using the formula: heat added = mass x specific heat capacity x temperature change.
Specific heat capacity is usually measured using a calorimeter, which measures the heat exchanged between a substance and its surroundings.
Correct, specific heat capacity is commonly determined using a calorimeter, a device specifically designed to measure the heat transfer of a substance. The calorimeter is able to calculate the specific heat capacity by measuring the temperature change of the substance and its surroundings as heat is exchanged. This allows for an accurate determination of the specific heat capacity of the substance being tested.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments