Science Matter Worksheets
Are you searching for engaging and educational worksheets that focus on the topic of matter in science? Look no further! These science matter worksheets are designed to captivate young minds and provide a solid foundation in understanding the properties and changes of different types of matter. Whether you are a teacher looking for classroom resources or a parent interested in supplementary material for your child, these worksheets are the perfect tool to ignite curiosity and knowledge about this captivating scientific subject.
Table of Images 👆
- Physical and Chemical Properties Worksheet Answer Key
- Science Properties of Matter Worksheets
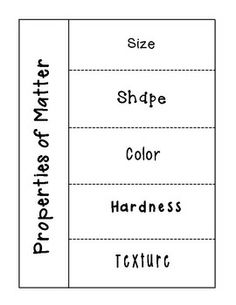
- Properties of Matter Foldable
- 3rd Grade Science Worksheets
- Scientific Method Worksheet 4th Grade
- Solar System Game Board
- Physical vs Chemical Change Worksheet
- Evaporation and Condensation Worksheets
- Glencoe Chemistry Chapter Assessment Answers
- 3rd Grade Math Assessment Test
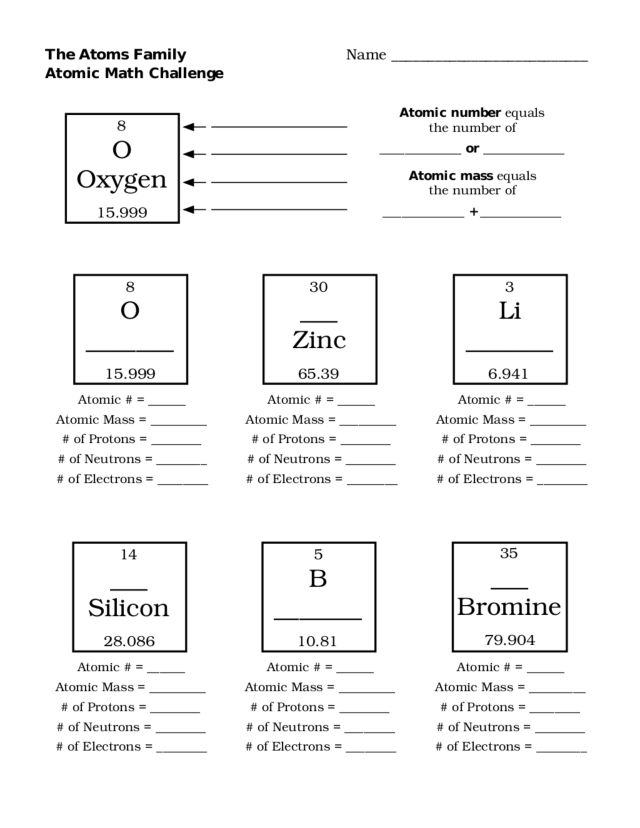
- Atoms Family Atomic Math Challenge Worksheet
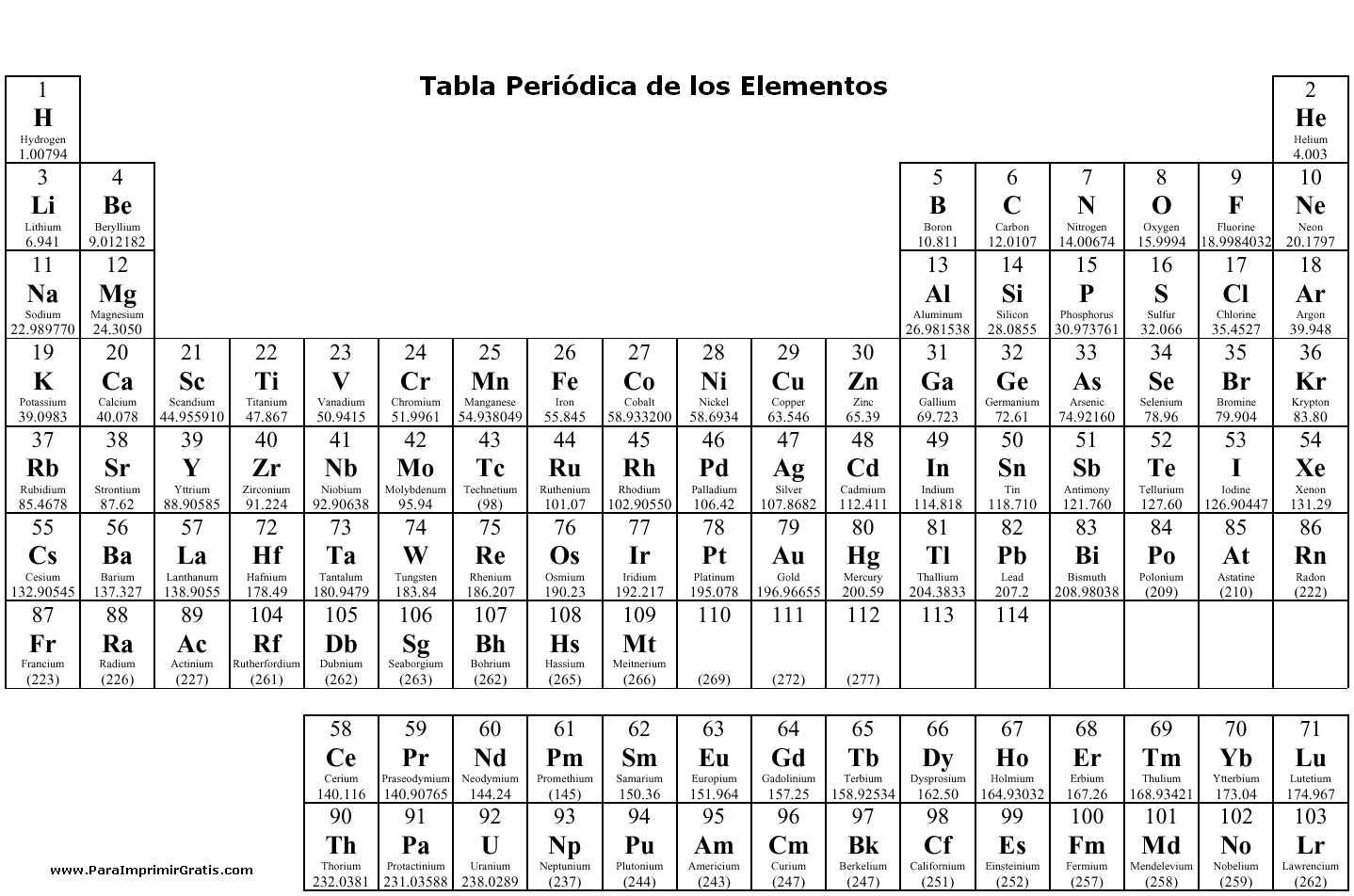
- Periodic Table with Element Charges
- Worksheets Answer Key
More Science Worksheets
6 Grade Science WorksheetsScience Heat Energy Worksheets with Answer
Science Worksheets Light and Sound
1st Grade Life Science Worksheets
7th Grade Science Cells Worksheets
Worksheets Life Science Vocabulary
8th Grade Science Scientific Method Worksheet
Science Worksheets All Cells
5th Grade Science Mixtures and Solutions Worksheets
What is the definition of matter?
Matter is anything that has mass and takes up space, consisting of atoms and molecules that are the building blocks of all substances in the universe.
How is matter classified?
Matter is classified into different categories based on its physical and chemical properties. One common classification is into elements, which are substances made up of only one type of atom; compounds, which are substances made up of two or more different types of atoms chemically bonded together; and mixtures, which are combinations of two or more substances that are not chemically bonded. Additionally, matter can also be classified as solids, liquids, or gases based on their physical state.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
Describe the properties of a solid.
A solid has a definite shape and volume, meaning it maintains its shape and does not change easily. It has a fixed arrangement of particles that vibrate in place but do not move freely. Solids are characterized by their high density and typically have strong intermolecular forces holding their particles together. They do not flow easily and have a rigid structure, making them resistant to compression.
What are the properties of a liquid?
Liquids have distinct properties, such as being incompressible, taking the shape of their container, having a fixed volume, being able to flow and diffuse, having a relatively high density compared to gases, and having a free surface. They also have a definite mass and can be easily poured or transferred from one container to another.
Explain the behavior of gases.
Gases have the ability to expand to fill their containers, exhibit low viscosity, compressibility, and density compared to solids and liquids. They also diffuse rapidly to evenly distribute themselves throughout a container, exert pressure on their surroundings, and can be cooled or heated to change their volume and pressure. Gas particles are in constant, random motion, colliding with each other and the walls of their container, leading to their unique behaviors.
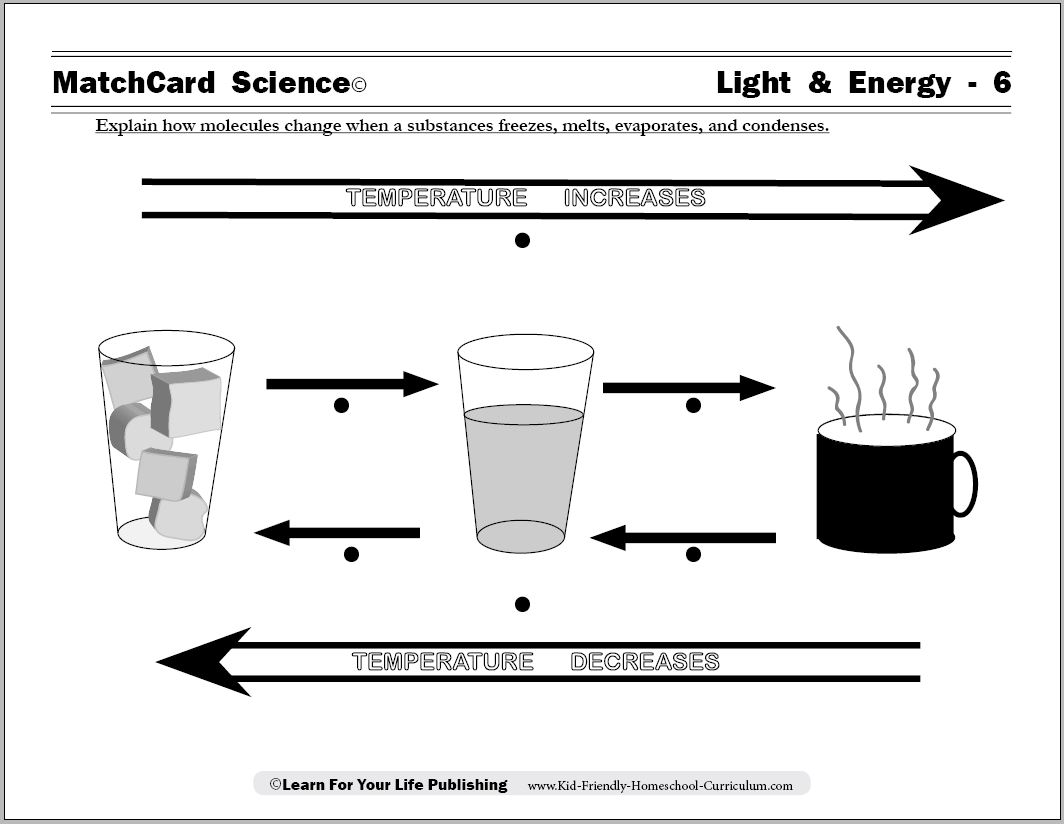
How does temperature affect the states of matter?
Temperature affects the states of matter by influencing the kinetic energy of the molecules within a substance. When temperature increases, the molecules move faster, overcoming the forces holding them together, and causing a change in state. For example, as a solid is heated, its particles gain more energy and move more freely, transitioning into a liquid. Further heating can lead to the liquid turning into a gas as the molecules move even faster. Conversely, decreasing temperature can cause a gas to condense into a liquid, and a liquid to solidify into a solid.
What is the difference between a mixture and a pure substance?
A mixture is a combination of two or more substances that are physically mixed together but not chemically bonded, allowing them to retain their individual properties. On the other hand, a pure substance is made up of only one type of particle, either atoms or molecules, and has a fixed composition and distinct set of properties. In short, mixtures can be separated into their individual components, while pure substances cannot be easily separated into different substances.
What is an element?
An element is a substance that cannot be broken down into simpler substances by chemical means. It consists of only one type of atom, each with a unique number of protons in its nucleus, giving it distinct chemical properties. Elements are fundamental building blocks of all matter in the universe, and they are organized in the periodic table based on their atomic number and properties.
Describe the concept of density in relation to matter.
Density is a physical property that explains how much mass is contained within a given volume of a substance. It is a measure of how tightly packed the particles of matter are in a substance. The higher the density, the more mass is present in a specific volume, indicating that the particles are more closely packed together. Density can help determine the behavior and characteristics of different materials, as it influences properties such as buoyancy, weight, and volume.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments