Protons Neutrons Electrons Table Worksheet
If you're a science teacher or student searching for a comprehensive and clear worksheet on protons, neutrons, and electrons, then this blog post is for you. In this worksheet, we will delve into the fascinating world of subatomic particles, their properties, and how they contribute to the overall composition of an atom.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
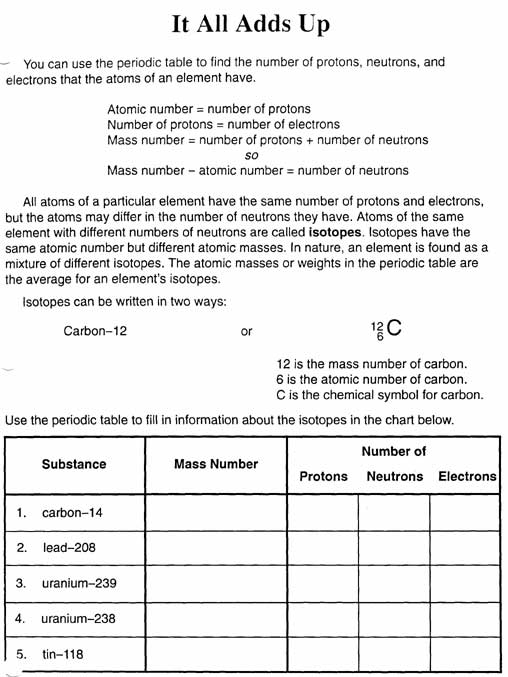
What is a proton?
A proton is a subatomic particle found in the nucleus of an atom with a positive electric charge. It is composed of two up quarks and one down quark bound together by the strong nuclear force, and its relative mass is approximately 1 atomic mass unit. Protons determine the chemical element of an atom and play a crucial role in the structure and stability of matter.
What is a neutron?
A neutron is a subatomic particle found within the nucleus of an atom that carries no electric charge. Neutrons, along with protons, make up the nucleus of an atom, and their presence helps to determine the stability and properties of an atom.
What is an electron?
An electron is a subatomic particle that carries a negative electric charge and is a fundamental component of matter. It is found in the outer regions of an atom and plays a crucial role in determining an atom's chemical properties, as well as participating in various interactions with other particles.
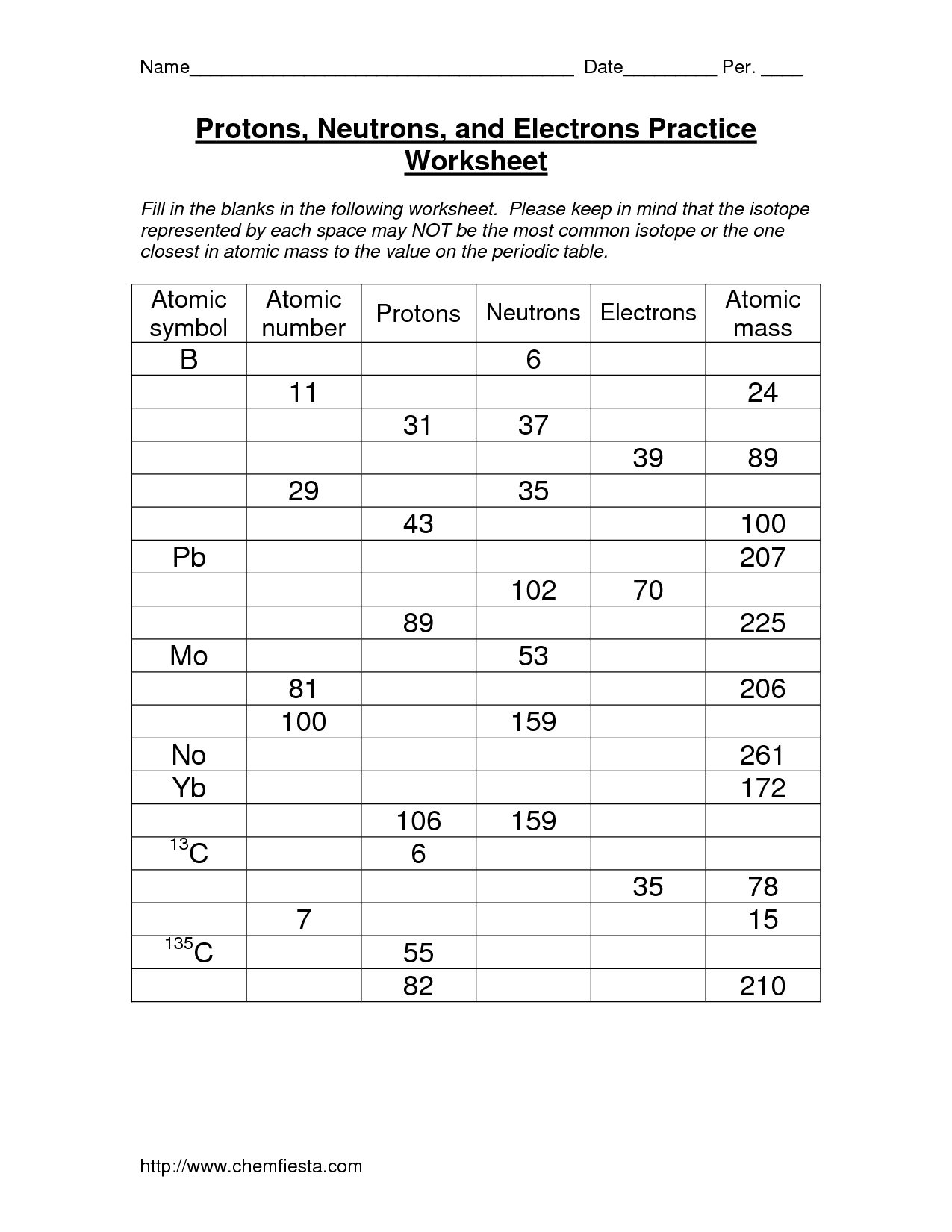
How are protons and neutrons different from electrons?
Protons and neutrons are located in the nucleus of an atom, have roughly the same mass (protons have a positive charge while neutrons are neutral), and are involved in determining the element's identity and stability. Electrons, on the other hand, are much smaller in mass, have a negative charge, and are found orbiting the nucleus. They play a key role in chemical reactions and bonding between atoms.
What is the charge of a proton?
The charge of a proton is +1 elementary charge, which is approximately 1.602 x 10^-19 coulombs.
What is the charge of a neutron?
A neutron has no charge, as it is composed of one up quark and two down quarks, which cancel out each other's charges, resulting in a neutral overall charge for the neutron.
What is the charge of an electron?
The charge of an electron is -1.6 x 10^-19 coulombs.
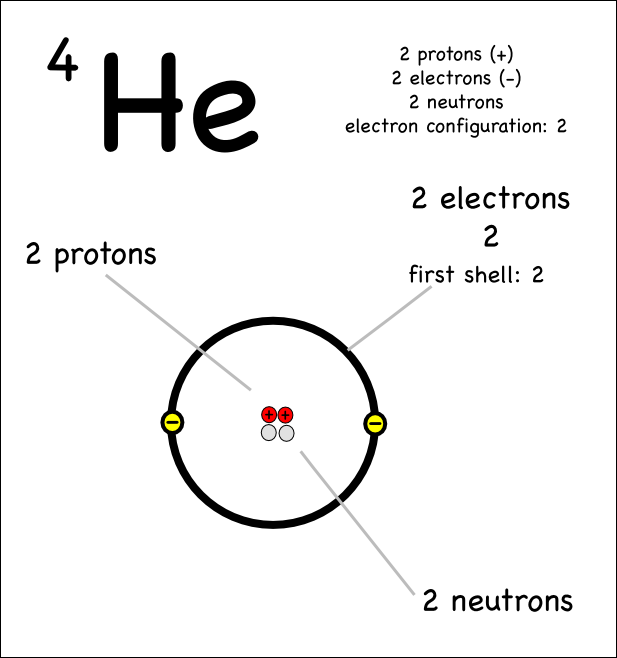
Where are protons and neutrons located in an atom?
Protons and neutrons are located in the nucleus of an atom, which is the central core of the atom. The nucleus is surrounded by a cloud of electrons that orbit the nucleus in specific energy levels or shells.
Where are electrons located in an atom?
Electrons are located in specific energy levels or shells surrounding the nucleus of an atom.
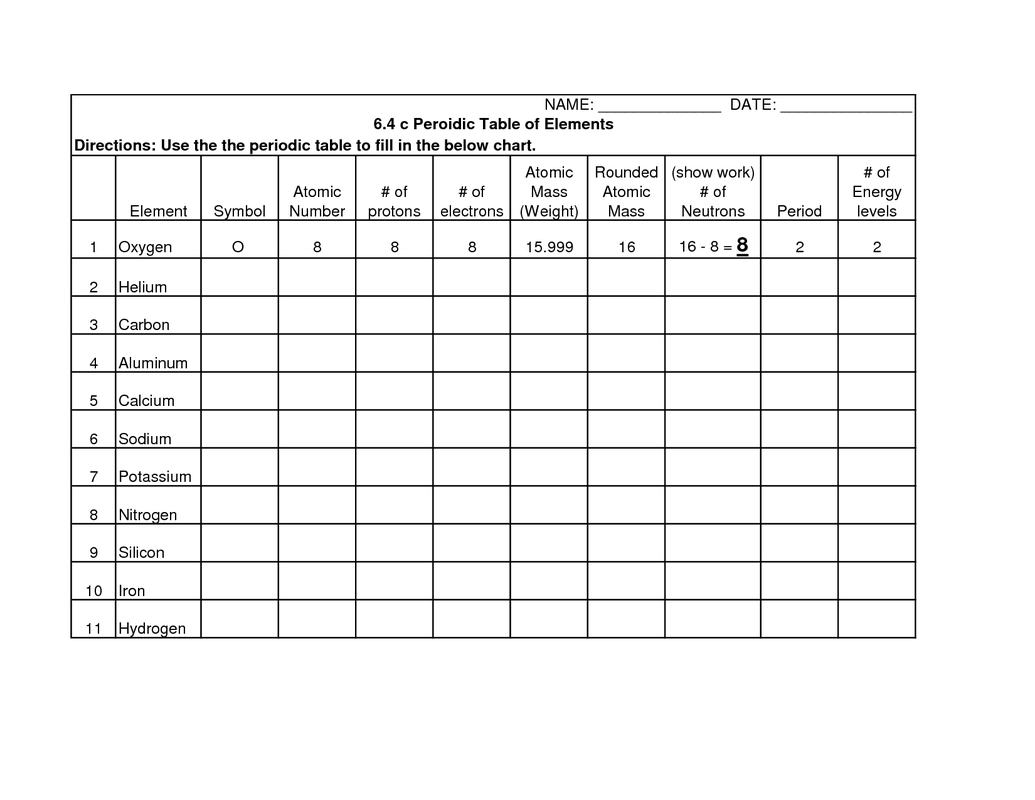
What is the role of protons, neutrons, and electrons in determining the properties of an element?
Protons determine the atomic number and positive charge of an element, while neutrons contribute to the atomic mass and stability of the nucleus. Electrons are responsible for the element's chemical behavior, bonding with other elements to form compounds. The arrangement of electrons in orbitals around the nucleus determines an element's physical and chemical properties, such as reactivity, conductivity, and ability to form ions or molecules. In summary, protons, neutrons, and electrons collectively determine the unique identity and properties of an element.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



















Comments