Prentice Hall Science Worksheets Chapter 5
The Prentice Hall Science Worksheets Chapter 5 provide an engaging and comprehensive resource for students who are studying science in their curriculum. These worksheets cover various topics within Chapter 5, presenting students with a wide range of exercises and questions to deepen their understanding of the subject matter. Whether you are a middle school student, a homeschooling parent, or a science tutor, these worksheets are designed to enhance the learning experience and help students excel in their science studies.
Table of Images 👆
- Biology Worksheet Answers Chapter 11
- Study Guide Science Chapter 6 2 Grade
- Pearson Biology Workbook a Answer Key Chapter 16
- Prentice Hall Chemistry Worksheet Answers
- Prentice Hall Physical Science Worksheets Answers
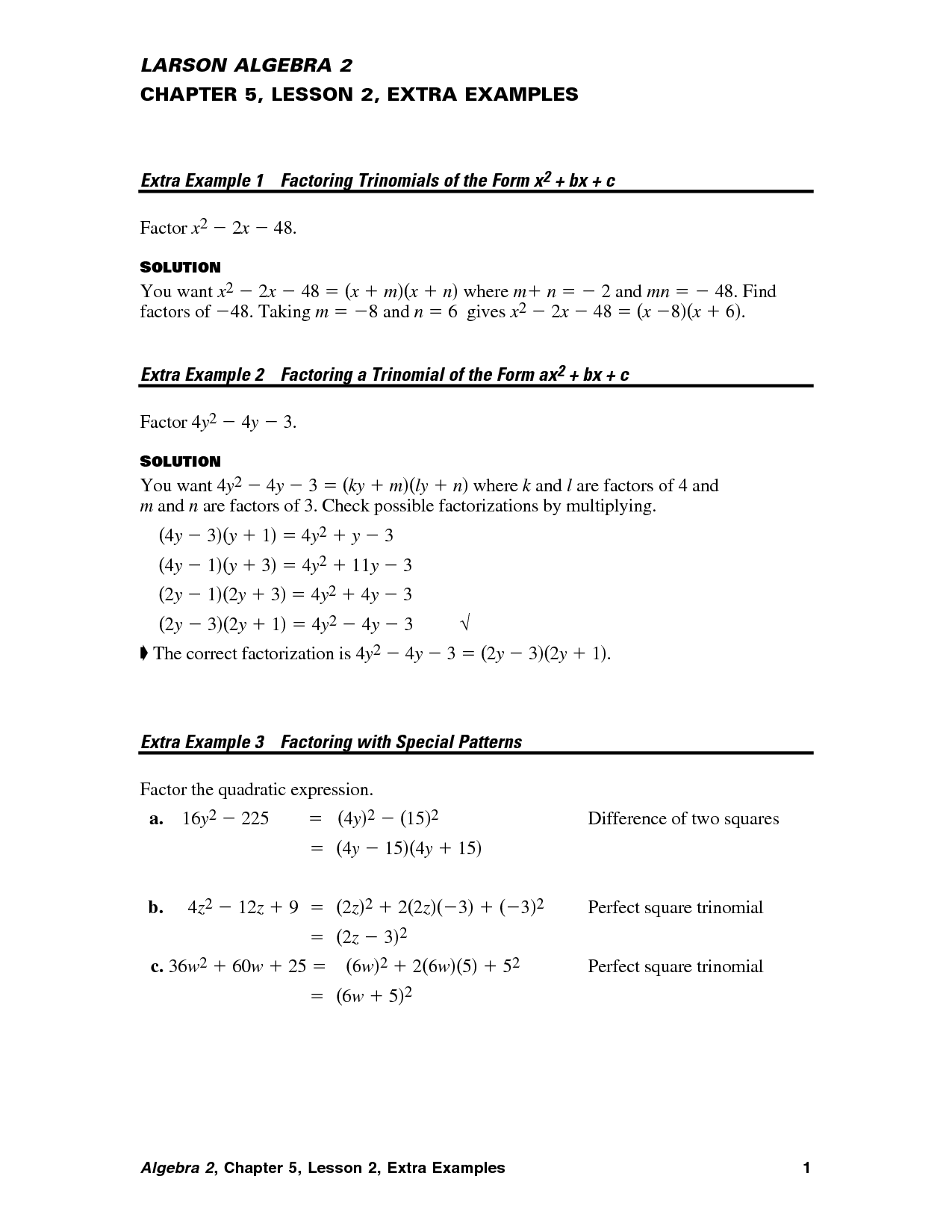
- Prentice Hall Algebra 2 Extra Practice Chapter 5
- Prentice Hall Chemistry Chapter 7 Assessment Answers
- Pearson Education Biology Worksheet Answers
- American Government Chapter 2 Worksheet Answers
- Glencoe Chemistry Chapter Assessment Answers
- Theory Law Worksheet Middle School
- Physical Science Chapter 1 Test Answers
- Chapter 12 Biology Answer Key
- Prentice Hall Biology Chapter 4 Test
- Prentice Hall Algebra 1 Chapter 8 Answers
- Science Study Guide Answers Chapter 4
More Science Worksheets
6 Grade Science WorksheetsScience Heat Energy Worksheets with Answer
Science Worksheets Light and Sound
1st Grade Life Science Worksheets
7th Grade Science Cells Worksheets
Worksheets Life Science Vocabulary
8th Grade Science Scientific Method Worksheet
Science Worksheets All Cells
5th Grade Science Mixtures and Solutions Worksheets
What is the definition of a physical change?
A physical change is a type of change in which the form or appearance of matter is altered, but not its chemical composition. This means that the substance remains the same at a molecular level, even though its physical characteristics may have changed. Examples of physical changes include changes in state (such as melting, freezing, or evaporating) or changes in shape or size.
Give an example of a physical change.
An example of a physical change is boiling water. When water is heated to its boiling point, it changes from a liquid to a gas, but its chemical composition remains the same.
What is the definition of a chemical change?
A chemical change is a process that involves the transformation of one substance into a different substance with distinct chemical properties through the rearrangement of atoms and bonds, resulting in the formation of new molecules.
Give an example of a chemical change.
An example of a chemical change is the combustion of gasoline in a car engine. During combustion, gasoline molecules react with oxygen from the air to produce carbon dioxide, water vapor, and heat energy. This process results in the formation of new substances with different chemical properties than the original gasoline molecules, demonstrating a chemical change.
How can you tell if a chemical change has occurred?
You can tell if a chemical change has occurred by observing if there are any changes in color, formation of a precipitate, evolution of gas, release of heat or light, or if there are irreversible changes in the chemical composition or properties of the substances involved. These are all indicators that a chemical reaction has taken place, leading to the formation of new substances different from the original reactants.
What is the law of conservation of mass?
The law of conservation of mass states that in a closed system, mass is neither created nor destroyed, but only changes forms. This means that the total mass of the system remains constant before and after any chemical reaction or physical change. This principle is a fundamental concept in chemistry and physics, and it emphasizes the idea that matter can be transformed and rearranged but cannot be created or destroyed.
What are the three states of matter and their characteristics?
The three states of matter are solid, liquid, and gas. Solids have a definite shape and volume, with particles that are closely packed and have minimal movement. Liquids have a definite volume but take the shape of their container, with particles that are closer together but still able to move past each other. Gases have neither a definite shape nor volume, with particles that are far apart and have high energy leading to rapid, random movement.
What is the difference between an element and a compound?
An element is a substance made up of only one type of atom, such as oxygen or gold, while a compound is a substance made up of two or more different types of elements chemically combined in a fixed ratio, like water (H2O) or salt (NaCl). Elements cannot be broken down into simpler substances by chemical reactions, whereas compounds can be broken down into their constituent elements through chemical reactions.
What are the three subatomic particles found in an atom?
The three subatomic particles found in an atom are protons, neutrons, and electrons. Protons have a positive charge, neutrons are neutral, and electrons have a negative charge. These particles are essential for the structure and behavior of an atom.
What is the significance of the periodic table in understanding the properties of elements?
The significance of the periodic table in understanding the properties of elements lies in its organization based on the atomic number and electron configuration of each element. The table allows for the systematic arrangement of elements according to their similar chemical properties and trends in behavior. This organization helps to predict an element's physical and chemical properties, such as reactivity, atomic size, and electronegativity, based on its position in the table. It also provides valuable information on the relationships between elements and helps scientists study and comprehend the behavior of elements in various chemical reactions and environments.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
































Comments