Physical States of Matter Worksheet
Are you a science teacher or a student interested in learning about the different physical states of matter? If so, this worksheet is designed with you in mind. Created to provide a comprehensive understanding of the topic, this worksheet focuses on the properties and characteristics of solids, liquids, and gases.
Table of Images 👆
- Science States of Matter Worksheets
- Physical and Chemical Changes of Matter Worksheet
- Physical vs Chemical Change Worksheet
- Physical vs Chemical Properties Worksheet
- States of Matter Worksheet PDF
- States of Matter Lesson Plans
- Properties of Matter Foldable
- Science Properties of Matter Worksheets
- Matter Science Graphic Organizers
- Physical and Chemical Changes Worksheets
- Elements and Their Symbols Worksheet
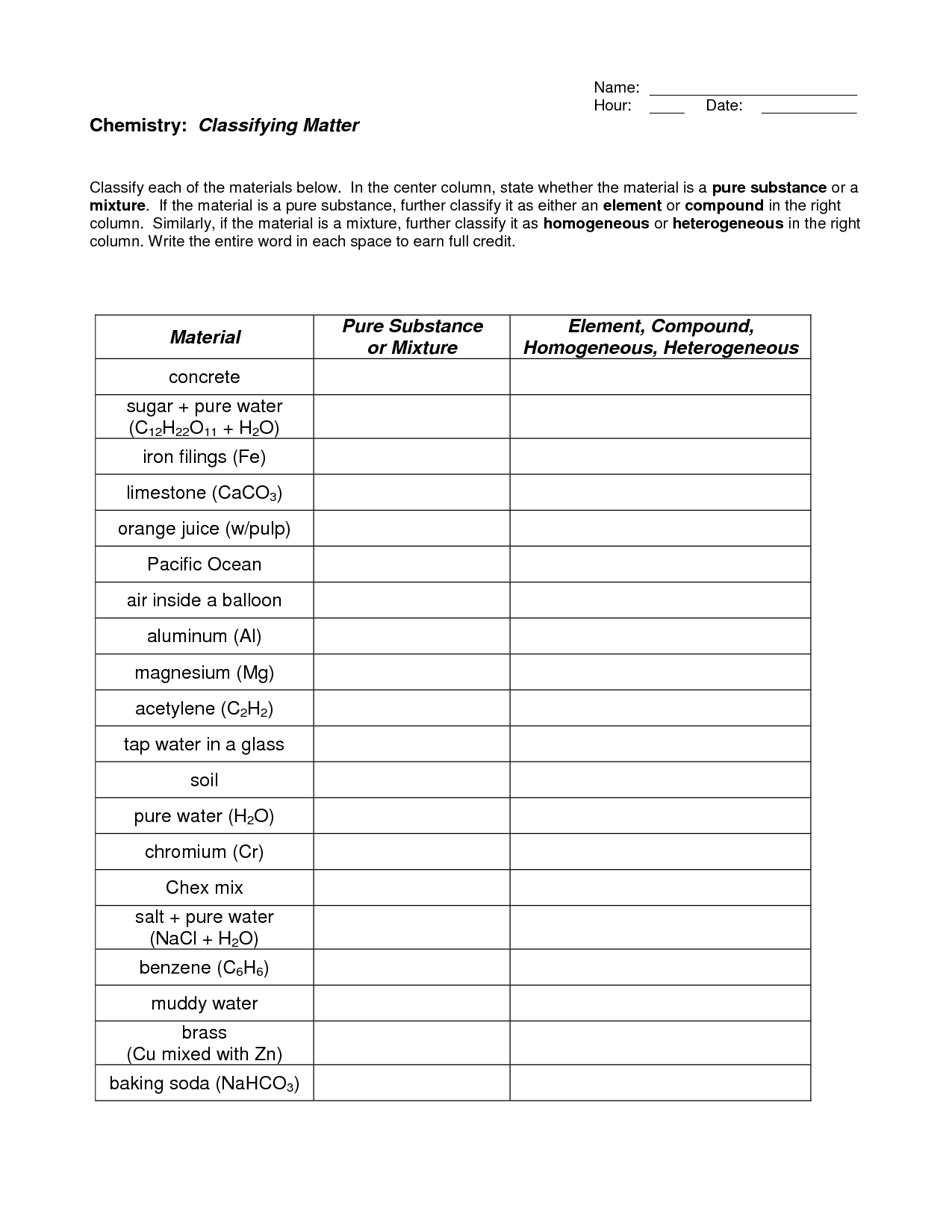
- Pure Substances and Mixtures Worksheet
- 3rd Grade Science Volume Mass Liquid
- States of Matter Worksheets 3rd Grade
- States Of Matter Worksheet Solid Liquid And Gas
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
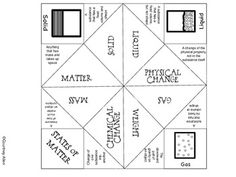
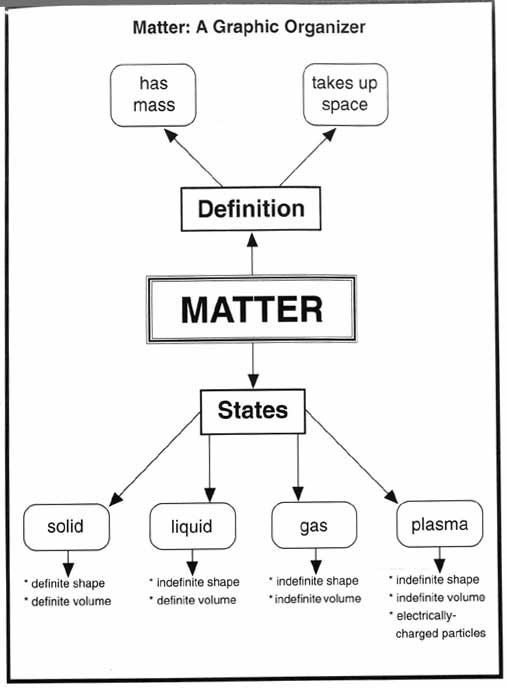
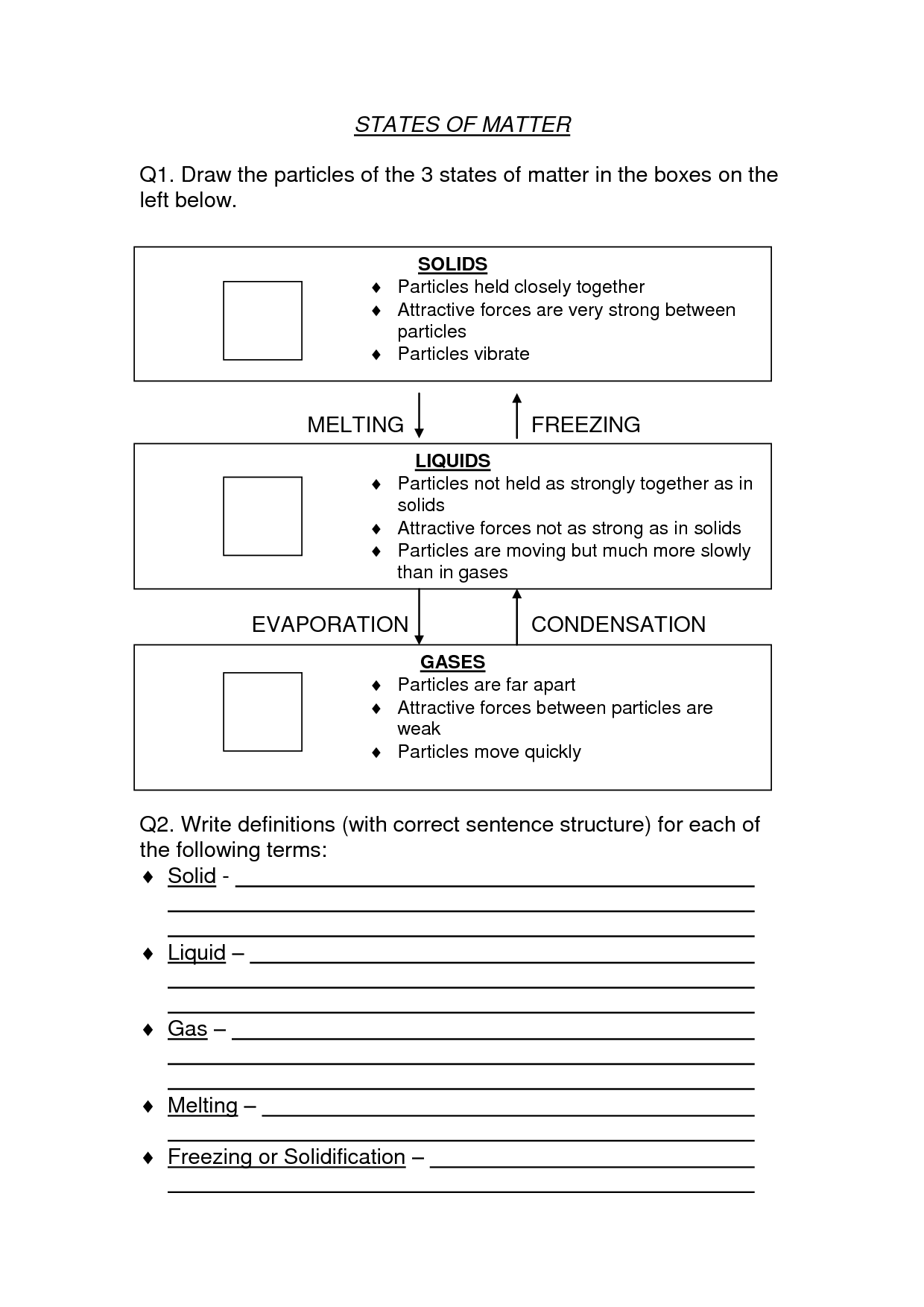
What is the physical state of matter in which particles are tightly packed together and have a definite shape and volume?
The physical state of matter in which particles are tightly packed together and have a definite shape and volume is a solid.
What physical state of matter has particles that are close together but can slide past one another, and has a definite volume but not a definite shape?
The physical state of matter that fits this description is a liquid. In a liquid, the particles are close together, allowing them to flow and slide past one another, giving liquids the ability to take the shape of their container while maintaining a definite volume.
What is the term for the physical state of matter in which particles are far apart, have no definite shape or volume, and can move freely and rapidly?
The term for the physical state of matter you are describing is "gas." In a gas, particles are widely spaced, have no fixed shape or volume, and move freely and quickly.
Name the physical state of matter that occurs when a substance transitions directly from a solid to a gas without passing through the liquid state.
The physical state of matter that occurs when a substance transitions directly from a solid to a gas without passing through the liquid state is called sublimation.
What is the term for the physical process in which a substance transitions from a gas to a solid without first becoming a liquid?
The term for the physical process in which a substance transitions directly from a gas to a solid without first becoming a liquid is called "deposition.
What physical state of matter usually has the highest density due to its tightly packed particles?
The physical state of matter that usually has the highest density due to its tightly packed particles is the solid state. The particles in a solid are closely packed together in a fixed arrangement, which results in strong intermolecular forces holding the particles in place. This close packing of particles leads to a higher density compared to liquids and gases, where the particles are more loosely packed and have weaker intermolecular forces.
At what temperature does a substance change from a solid to a liquid?
The temperature at which a substance changes from a solid to a liquid is known as its melting point. The melting point varies for different substances, as each material has its own unique melting point.
What is the term for the amount of energy required to raise the temperature of a substance and change its physical state?
The term for the amount of energy required to raise the temperature of a substance and change its physical state is specific heat capacity.
What happens to the energy of a substance as it changes from a solid to a liquid, and from a liquid to a gas?
As a substance changes from a solid to a liquid, energy is absorbed to break the intermolecular forces that hold the solid structure together, resulting in an increase in kinetic energy. This process is called melting. Similarly, when a substance changes from a liquid to a gas, energy is absorbed to break the intermolecular forces that hold the liquid together, resulting in increased kinetic energy. This process is called vaporization or evaporation. Overall, the energy increases as the substance transitions from a solid to a liquid, and from a liquid to a gas.
What is the term for the process in which a substance changes from a gas to a liquid or solid, releasing energy in the form of heat?
The term for the process in which a substance changes from a gas to a liquid or solid, releasing energy in the form of heat, is called condensation.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments