Periodic Trends Worksheet with Answers
Understanding the periodic trends in the elements of the periodic table can be a challenging task. If you are a student or an educator searching for a reliable resource to enhance your knowledge and comprehension of these trends, you have likely come across various worksheets. Worksheets provide a structured approach to tackle this complex subject, enabling you to practice and apply the concepts to reinforce your understanding. In this blog post, we will explore a periodic trends worksheet with answers, offering a valuable resource for those seeking to expand their knowledge of this essential topic in chemistry.
Table of Images 👆
- Periodic Trends Worksheet Answers
- Periodic Table Worksheet Answer Key
- Periodic Table Trends Worksheet Answer Key
- Periodic Trends Worksheet Answers
- The Periodic Table Elements and Atoms Worksheet Answers
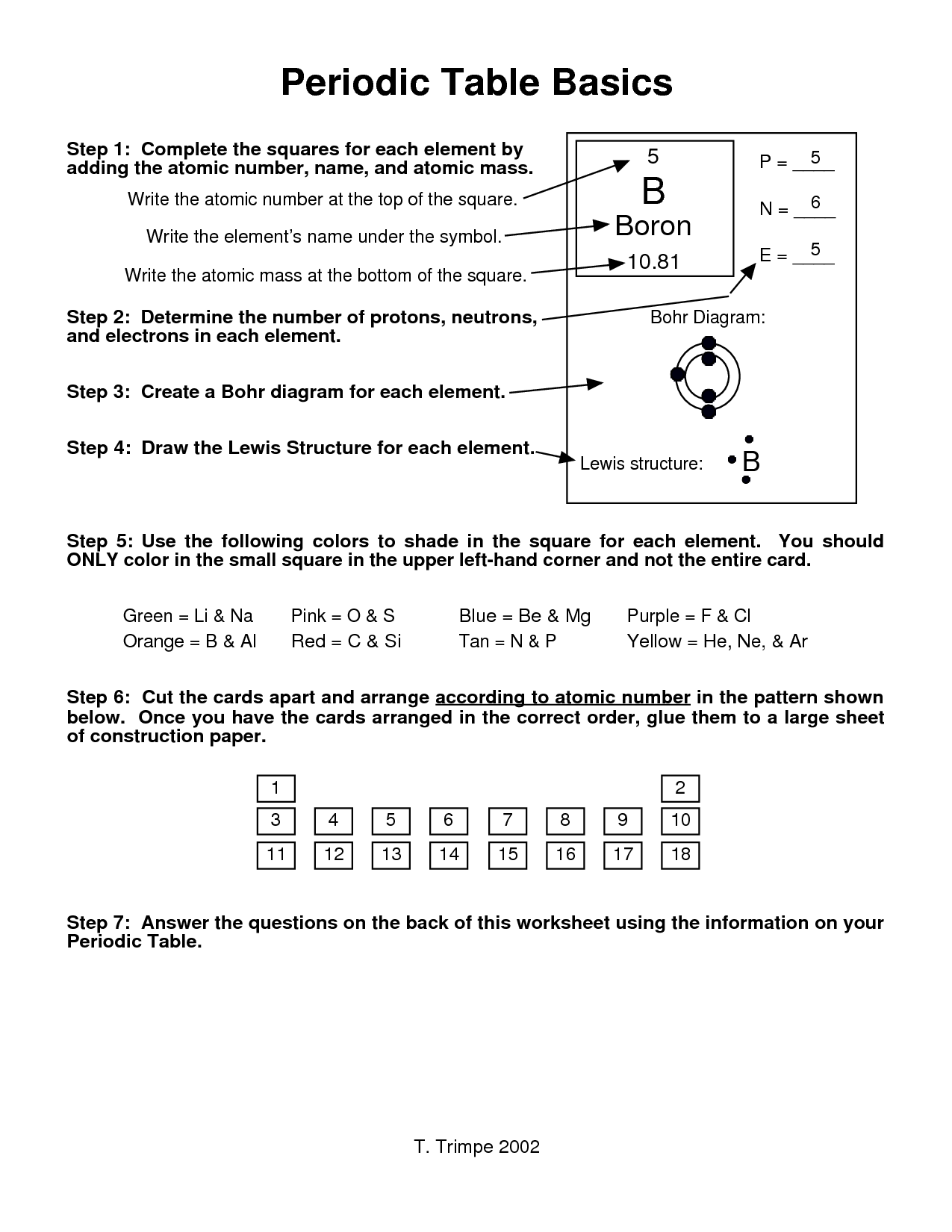
- Periodic Table Basics Worksheet Answers
- Chemical Reaction Worksheet Answer Key
- Blank Periodic Table
- Black and White Periodic Table
- Periodic Table of Elements Blank Worksheet
- Chemistry Study Guide Answer Key Chapter 2
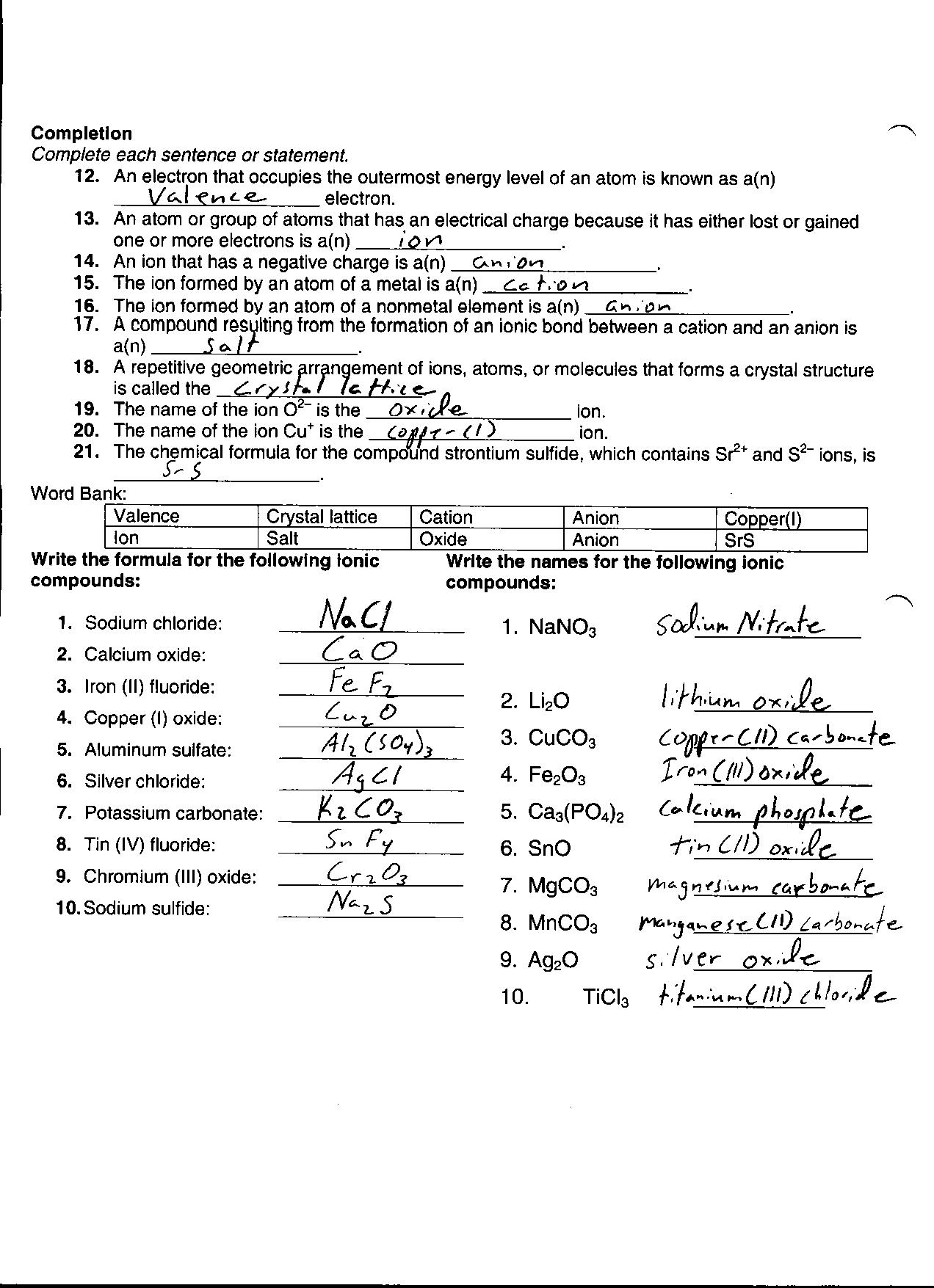
- Electron Configuration Worksheet Answers
- Electron Configuration Practice Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
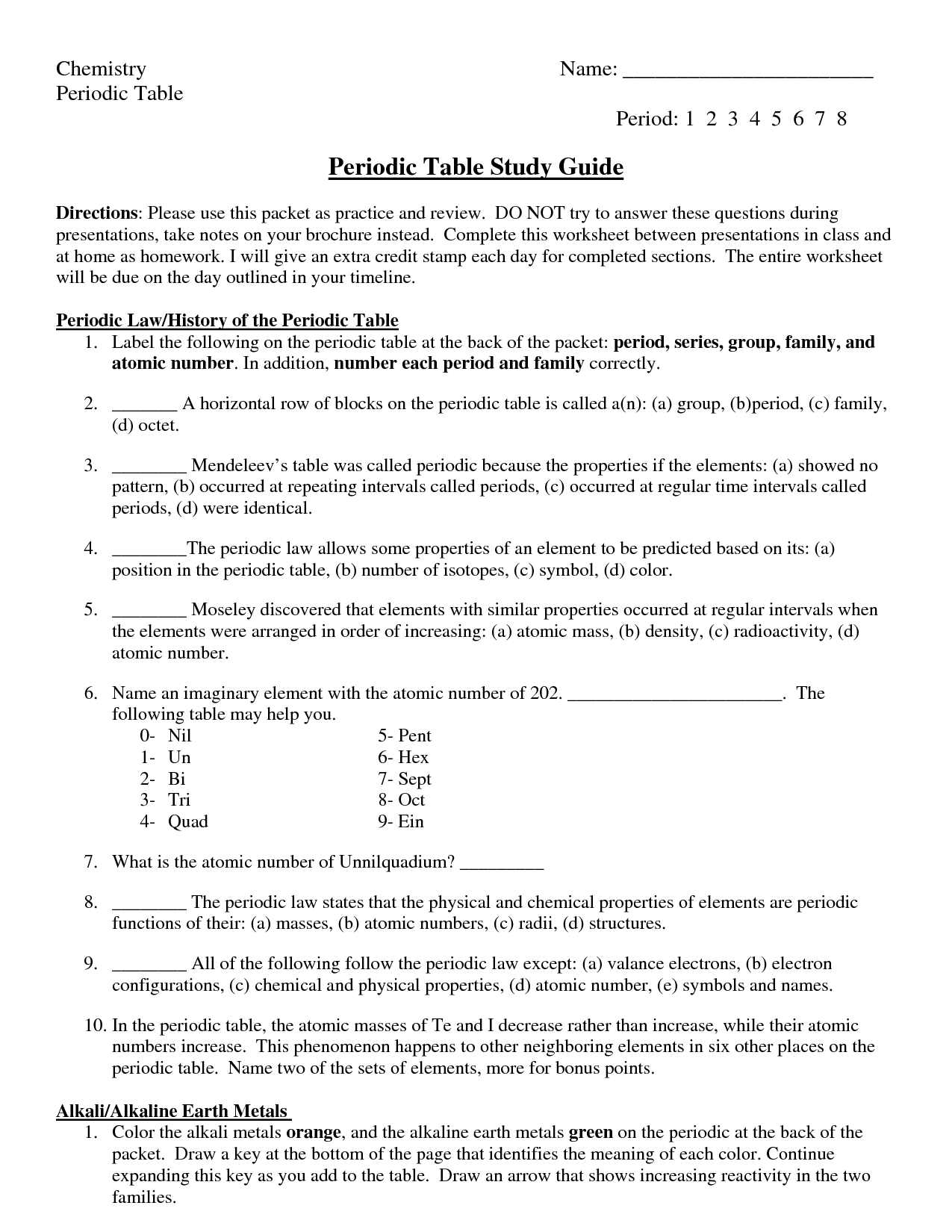
What is periodicity in the context of periodic trends?
Periodicity in the context of periodic trends refers to the consistent and predictable pattern in the properties of elements as you move across a period or down a group on the periodic table. These trends include variations in factors such as atomic radius, ionization energy, electron affinity, and electronegativity, which repeat in a regular and systematic manner. By understanding periodicity, scientists can predict the behavior and properties of elements based on their positions in the periodic table.
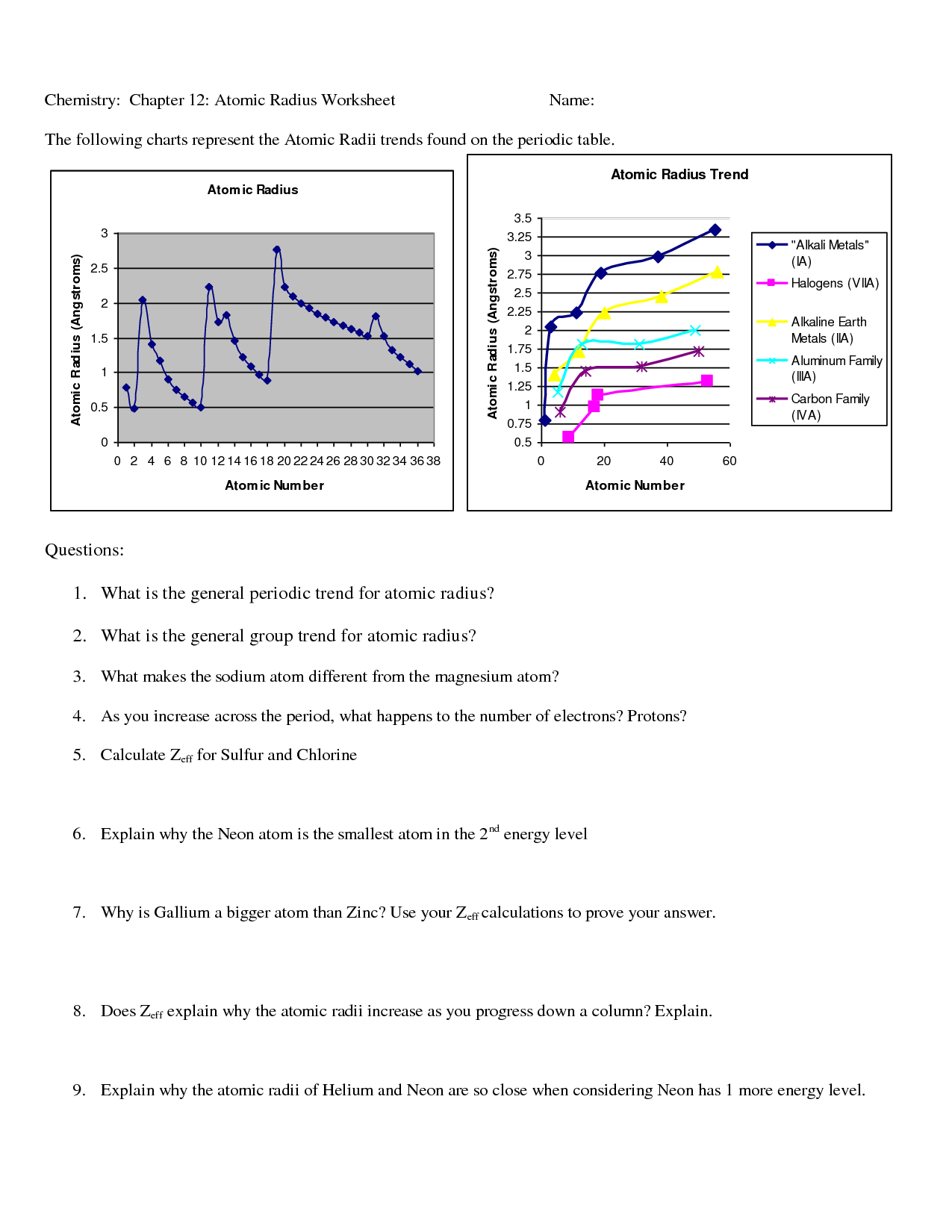
What is the periodic trend of atomic radius across a period?
The atomic radius decreases across a period from left to right. This trend is due to increasing nuclear charge as the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons, causing the electron cloud to be pulled closer to the nucleus and resulting in a smaller atomic radius.
What is the periodic trend of atomic radius down a group?
The periodic trend of atomic radius down a group is that the atomic radius increases as you move down the group. This increase in atomic radius is due to the addition of electron shells as you go from one period to the next, leading to larger atomic sizes.
How does ionization energy change across a period?
Ionization energy generally increases across a period from left to right. This trend is due to the increasing number of protons in the nucleus, which results in a stronger attraction for electrons in the outermost energy level. As a result, more energy is required to remove an electron from an atom as you move across a period.
How does ionization energy change down a group?
Ionization energy generally decreases down a group in the periodic table. This is because as you move down a group, the atomic size increases due to the addition of more energy levels. With the outer electrons being farther away from the nucleus, the effective nuclear charge decreases, resulting in weaker attraction between the nucleus and outer electrons. As a result, less energy is needed to remove an electron, leading to a decrease in ionization energy down a group.
What is the periodic trend of electronegativity across a period?
Electronegativity generally increases across a period from left to right. This trend is due to the increasing effective nuclear charge experienced by the outer electrons as the number of protons in the nucleus increases, leading to a greater ability to attract electrons towards the atom.
What is the periodic trend of electronegativity down a group?
The electronegativity of elements generally decreases down a group in the periodic table. This trend occurs because as you move down a group, the distance between the nucleus and the outermost electrons increases, leading to a weaker attraction between the nucleus and the electrons.
How does electron affinity change across a period?
Electron affinity generally increases across a period from left to right. This is because as you move across a period, the effective nuclear charge experienced by the outer electrons increases, making it easier for the atom to accept an additional electron.
How does electron affinity change down a group?
Electron affinity generally decreases down a group in the periodic table. This is because as you move down a group, the atomic size increases, leading to a weaker effective nuclear charge that is less able to attract additional electrons. As a result, atoms in lower periods have lower electron affinity values compared to those in higher periods within the same group.
What is the periodic trend of metallic character across a period?
The metallic character decreases across a period from left to right. This trend is due to increasing effective nuclear charge, which leads to stronger attraction between the valence electrons and the nucleus, making it harder for metallic elements to lose their electrons and exhibit metallic properties.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments