Periodic Table Practice Worksheet
Are you a student or teacher who is looking for a resource to help reinforce your understanding of the periodic table of elements? Look no further! A periodic table practice worksheet is a valuable tool that allows you to test your knowledge and become more familiar with the various elements and their properties. Whether you're studying chemistry or just interested in learning more about the elements, this worksheet can provide an engaging and interactive way to enhance your education.
Table of Images 👆
- Periodic Table Blank Worksheet
- Periodic Table Blank Worksheet
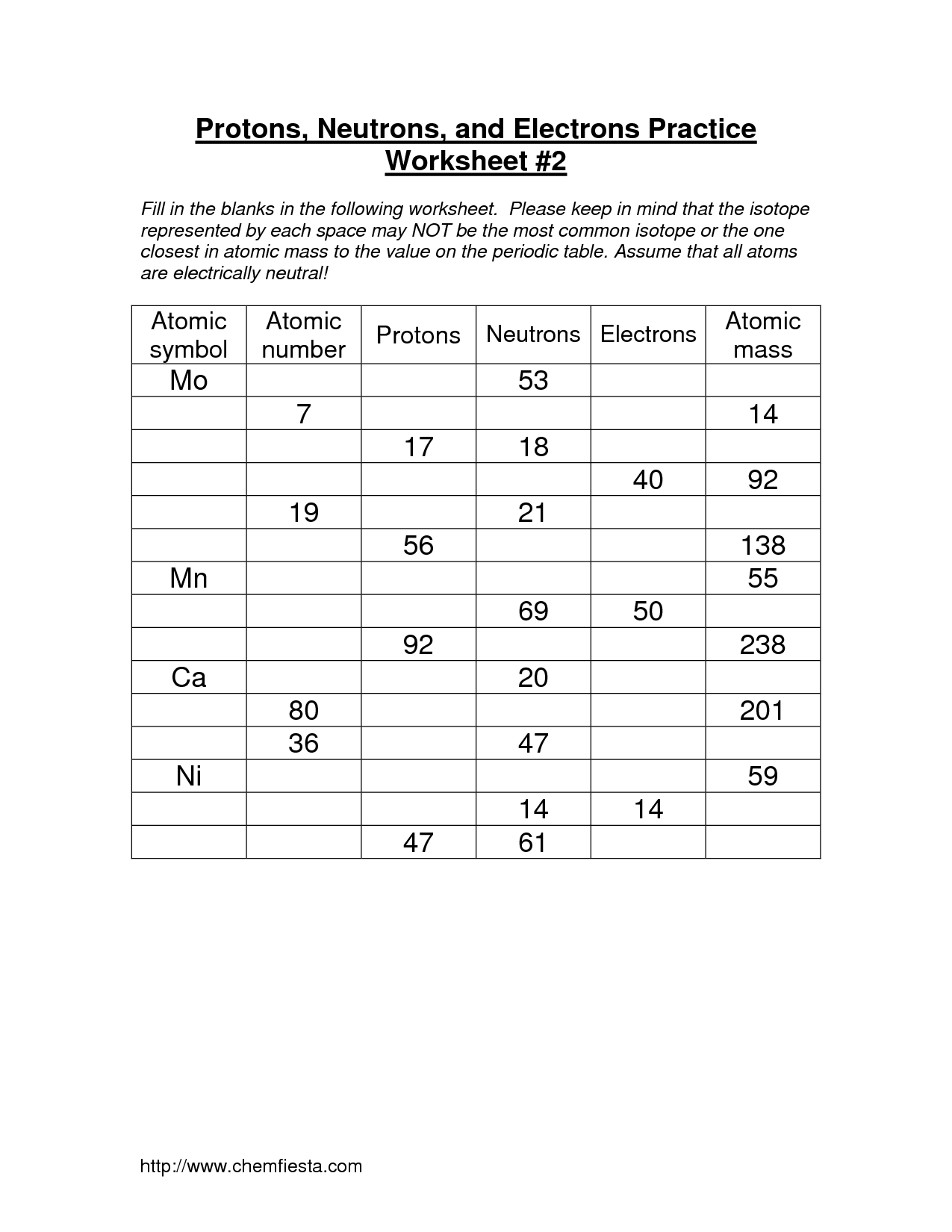
- Protons Neutrons Electrons Practice Worksheet Answers
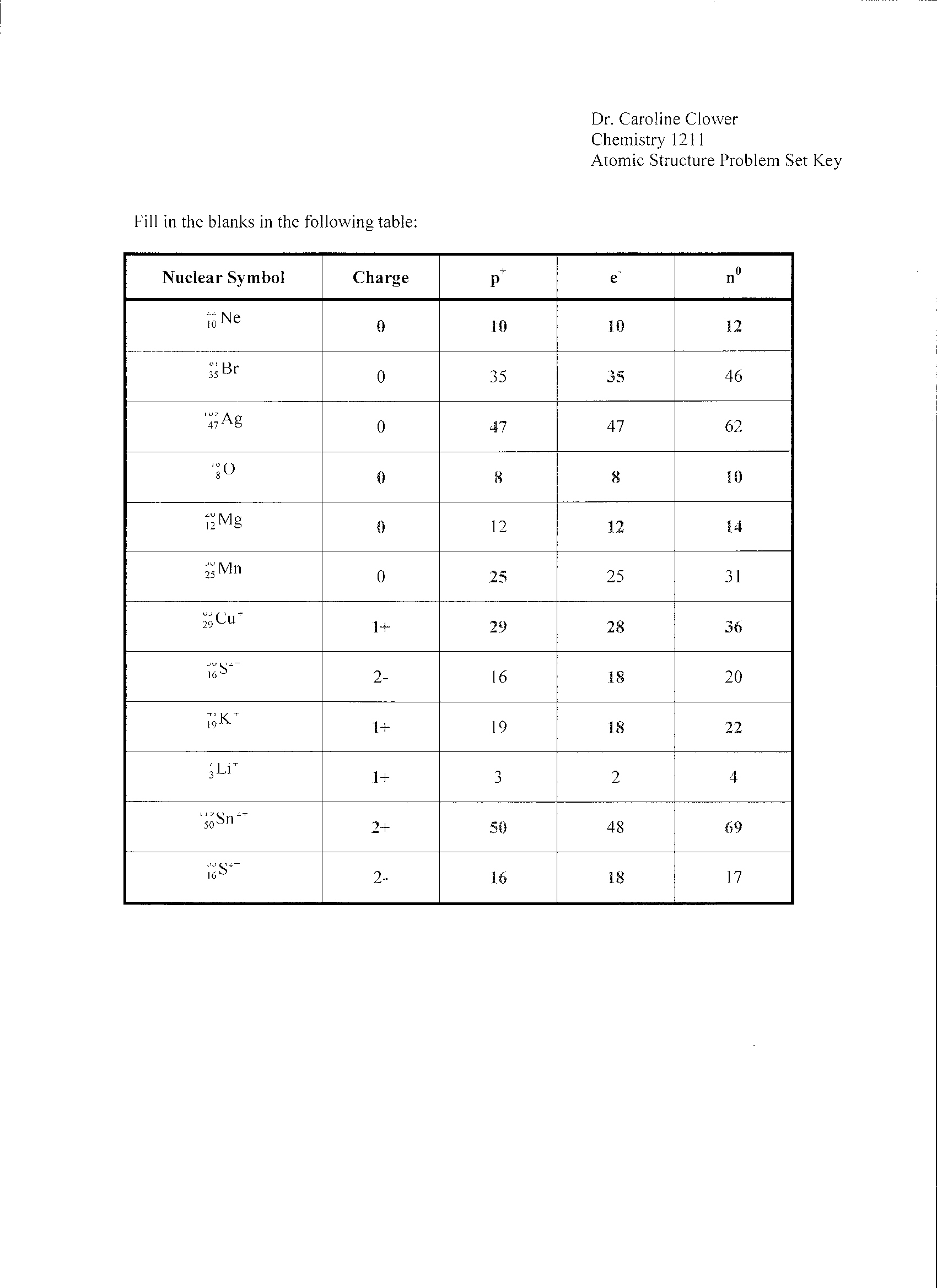
- Isotopes and Ions Practice Worksheet Answers
- Atomic Structure Worksheet Answers
- Periodic Trends Worksheet Answer Key
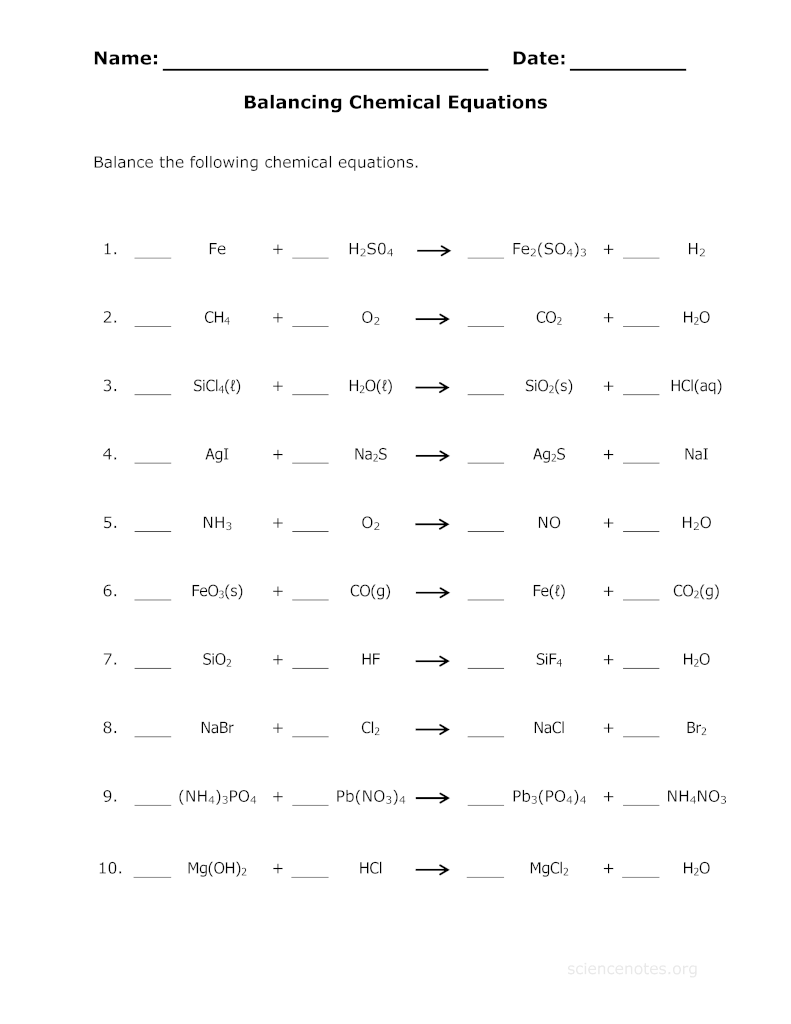
- Balancing Equations Practice Worksheet Answers
- Bonding Worksheet Answer Key
- Bohr Model Worksheet Answers
- Periodic Table Puns Worksheet Answers
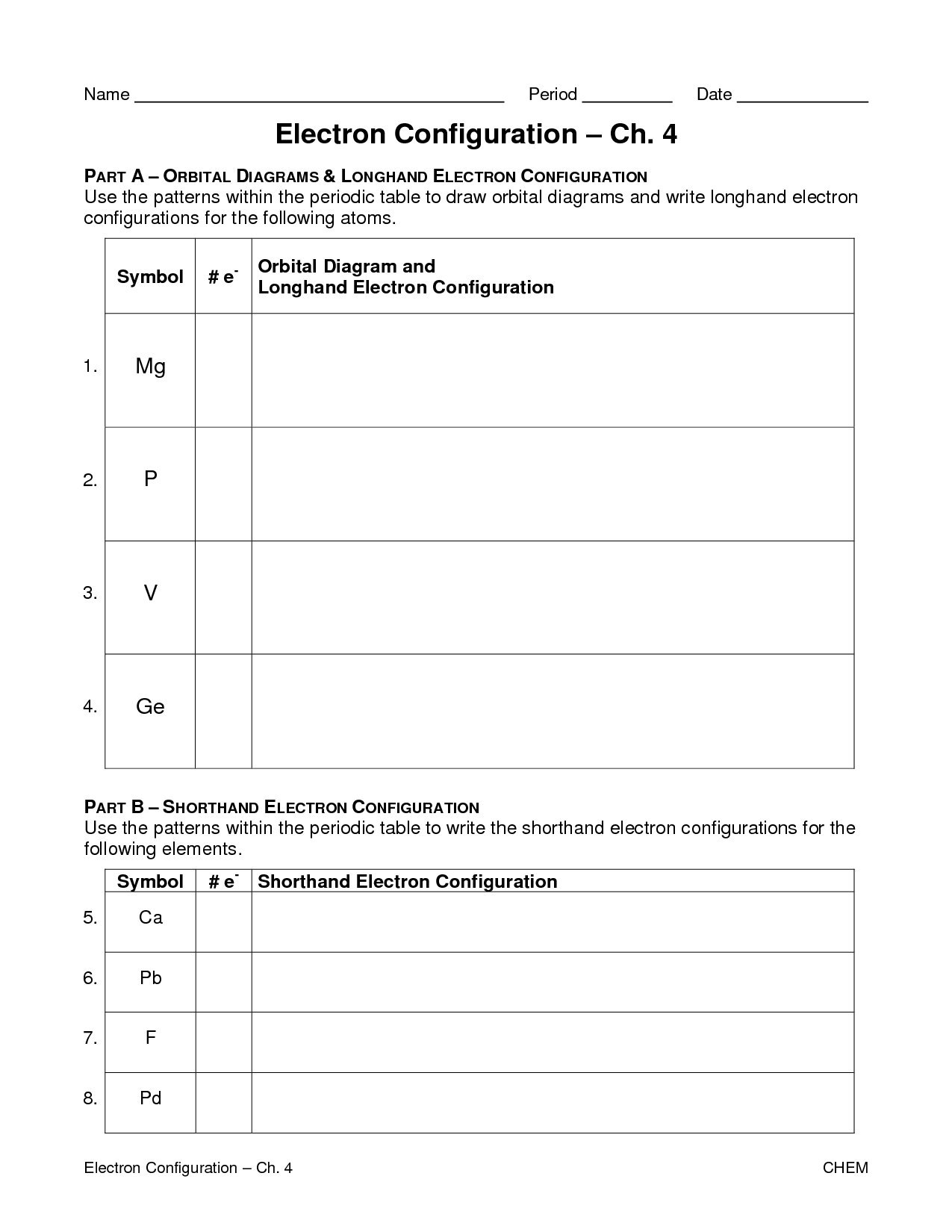
- Electron Configuration and Quantum Numbers Worksheet
- Electron Configuration Worksheet
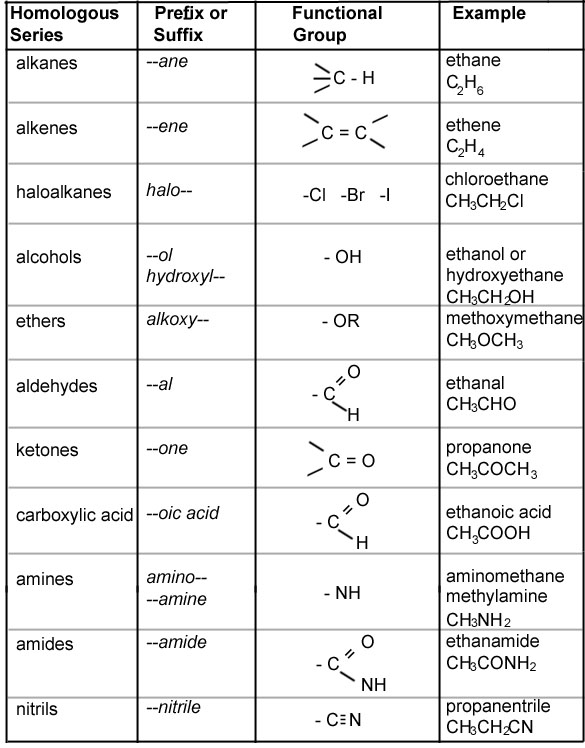
- Organic Chemistry Nomenclature
- Counting Atoms Worksheet Answers
- Counting Atoms Worksheet Answers
- Counting Atoms Worksheet Answers
- Counting Atoms Worksheet Answers
- Counting Atoms Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
What is the Periodic Table?
The Periodic Table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and chemical properties. It displays elements in rows and columns that illustrate recurring patterns in their properties and allows for easy identification of trends, relationships, and groupings among the elements.
How are elements organized on the Periodic Table?
Elements on the Periodic Table are organized by increasing atomic number, which represents the number of protons in the nucleus of an atom. The table is divided into periods (rows) and groups (columns), where elements in the same group have similar chemical properties due to the same number of valence electrons. Additionally, elements are classified as metals, nonmetals, or metalloids based on their physical and chemical properties, with a stair-step line separating metals from nonmetals on the Periodic Table.
What information can be found for each element on the Periodic Table?
For each element on the Periodic Table, you can find the element's name, symbol, atomic number, atomic weight, electron configuration, and the group and period to which it belongs. Additional information such as melting point, boiling point, density, and common uses of the element can also be found. The periodic table provides a comprehensive overview of the properties and characteristics of all known elements.
How many elements are currently known?
As of 2021, there are 118 known elements on the periodic table.
What are periods on the Periodic Table?
Periods on the Periodic Table are the horizontal rows that categorize elements based on the number of electron shells they have. Each period represents the number of electron shells an element's atoms possess, with the first period containing elements that have one electron shell and subsequent periods adding one more electron shell in each row.
What are groups or families on the Periodic Table?
Groups or families on the Periodic Table are vertical columns that contain elements with similar chemical properties. There are 18 groups in the Periodic Table, each with its own distinct characteristics and trends. Elements within the same group typically have the same number of valence electrons, which govern their chemical reactivity and behavior. Examples of groups include the alkali metals in group 1, the halogens in group 17, and the noble gases in group 18.
How are elements within a group similar to each other?
Elements within a group in the periodic table are similar to each other because they have the same number of valence electrons, which determines their chemical properties. This results in elements within the same group having similar reactivity, bonding characteristics, and general behavior in chemical reactions. Additionally, elements in the same group often share similar physical properties such as melting and boiling points, as well as atomic radius and ionization energy. Overall, elements within a group exhibit similarities due to their shared electronic configuration and position in the periodic table.
How are elements within a period similar to each other?
Elements within a period are similar to each other in that they have the same number of electron shells. This means that they have similar atomic sizes and reactivity patterns. Additionally, elements within a period often exhibit a gradual change in properties as you move across the periodic table due to the increase in the number of protons and electrons.
What is the significance of the atomic number for each element?
The atomic number of an element represents the number of protons found in the nucleus of its atoms, which in turn determines the element's unique chemical and physical properties. It defines an element's identity on the periodic table and dictates its placement within the table. The atomic number also plays a crucial role in determining the element's reactivity, bonding behavior, and overall characteristics, making it a fundamental concept in the field of chemistry.
What are the different types of elements found on the Periodic Table?
The Periodic Table consists of different types of elements such as metals, nonmetals, and metalloids. Metals are usually solid at room temperature, conductive, malleable, and ductile. Nonmetals are generally poor conductors, and many are gases or brittle solids. Metalloids share properties of both metals and nonmetals, exhibiting characteristics such as semiconductivity.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments